75 years of Grünenthal
Letter from Gabriel Baertschi
CEO Grünenthal
This year, Grünenthal celebrates 75 years of developing breakthrough medicines for patients around the world. For us, it is a time to take stock of everything we have achieved over the last eight decades to move closer towards our vision of a world free of pain.
Since it was founded in 1946, Grünenthal has made enormous progress and has developed into a global organisation with over 4,500 employees working across Europe, Latin America and the USA.
“I could not be prouder of our company.”
This is certainly cause for celebration. However, we will not forget the difficult moments from our story. The Thalidomide tragedy has been part of our history since 1957 – and always will be. With the Grünenthal Foundation, we are contributing to improving the individual daily living situations of people affected by Thalidomide.
We continue to work on improving the lives of patients and their families.
In particular, I would like to thank our employees for ensuring an uninterrupted supply of our medicines for patients during the pandemic.
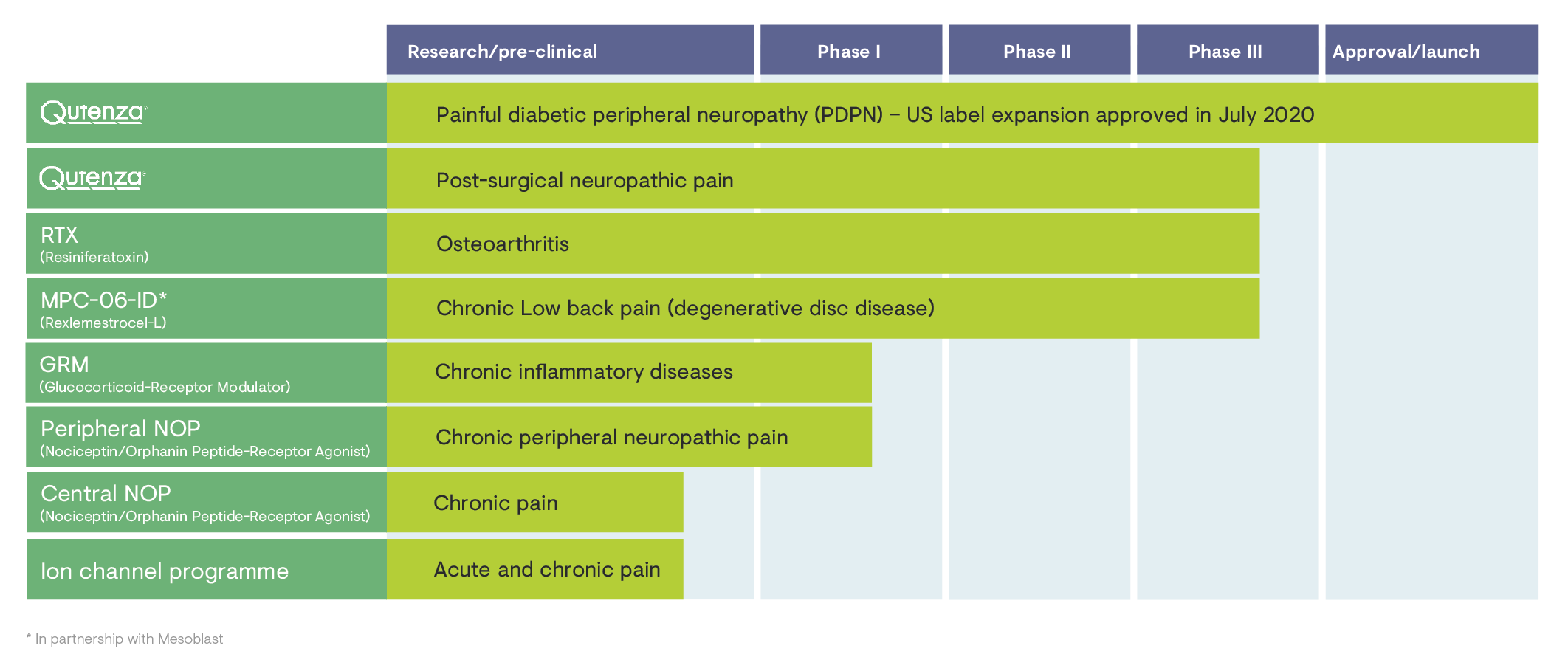
In recent years, we have further developed our pipeline. It now includes three projects in Phase III and two projects in Phase I of clinical development. Our research teams are working on programmes involving a range of innovative mechanisms of action and different modalities, including cell therapy. In R&D, we have strengthened our own expertise in pain research, as well as our collaboration with innovative partners.
The work of our scientists in Aachen and in our Innovation Hub in Boston underscores our commitment to developing urgently needed medications for pain therapy.
Today, Grünenthal products are available in over 100 countries around the world. To secure our market position, we have invested over €1.3 billion in acquisitions and expanded our portfolio with established brands. The continuing success of our own products and our focused in-licencing approach have contributed to a strengthened EBITDA, which reached record levels in 2019. Following our early expansion into Latin America, Grünenthal entered the market in the USA in 2018. With partnerships on every continent, we are now a truly global company.
In the future development of Grünenthal, our employees and our company culture will continue to play a key role in our success. For this reason, we will invest in the capabilities of our teams, drive digitalisation in our company, expand our portfolio and drive profitability without compromising on safety or quality.
On behalf of the Corporate Executive Board, I would like to thank everyone at Grünenthal for their tireless dedication to bringing innovative treatments to patients worldwide. I could not be prouder of the company we have built, and I am excited about the next few years as we pursue our vision: a world free of pain.


Letter from Prof. Dr. Wilhelm Moll LL.M.
Chairman of the Supervisory Board of Grünenthal
The year 1946, when “Chemie Grünenthal” was founded, marked a political, social and economic new beginning on almost every level. Post-war Germany faced difficult years ahead. Those who demonstrated pioneering spirit at that time would end up taking part in the economic miracle in later years. The permission granted by the North Rhine-Westphalian state government in 1948 for the production of penicillin was also intended to pave the way for Grünenthal to provide patients worldwide with important medicines. However, looking back over its 75 years of existence, Grünenthal can state today that the pioneering spirit of that time and the clear focus on innovation have remained the guiding principles for the successful development of the company.
Grünenthal’s history has involved highs and lows. We are still working continuously to coordinate our response to the Thalidomide tragedy and all of its devastating consequences. The company is still deeply affected by the suffering that Thalidomide caused for affected people and their families. We are convinced that the Grünenthal Foundation for the Support of Thalidomide-affected People represents the right path for us to take together with the people affected. The support that we offer through the Foundation for the everyday life and living conditions of those affected will continue to be adapted to meet changing needs in the future, as it has been in the past.
“The employees are the foundation of this success.”
In the past decades, Grünenthal has achieved a leading position in the field of pain and provides medicines for millions of patients. This rise has been shaped by many transformations and changes. Our clear commitment to research and development has produced innovative medicines and advanced Grünenthal’s development over the years. Grünenthal’s employees are the foundation of this success. They always have the patient in mind and work hard every day to keep moving closer towards the vision of a world free of pain.
For this, they deserve a great deal of thanks.
As a family business, Grünenthal is characterised by the cooperative interaction of shareholders, the advisory board, management and employees. The successful and forward-looking development of recent years would not have been possible without the trustbased cooperation of everybody involved. The continuation of this constructive cooperation gives the company a promising and stable outlook for the future, even in a changing world.


Letter from Sibylle Keupen
Mayor of the City of Aachen
On behalf of the entire Council of the City of Aachen and the administration, I congratulate all employees as well as the management and shareholders of Grünenthal on the company’s 75th anniversary. There is an eventful history behind your company. In particular, the story of the post-war years in West Germany cannot truly be told without mentioning Grünenthal.
“An important partner for our region.”
The fact that a business from Stolberg was the first West German company to succeed in producing penicillin in large quantities is just one example of the entrepreneurial spirit and innovative strength with which Grünenthal has maintained its international position to this day. Patient-centric innovations that are made in Aachen are researched here, which contributes to the role of our region as an important global centre for science. However, Grünenthal’s economic significance and its role as an employer and trainer are also factors that make Grünenthal an important partner for us in the region.
For the future, I wish all employees continued success and hope that you are able to carry the commitment and innovative spirit of the last 75 years into a new era. Our society faces many challenges. Your work is of the greatest importance for millions of patients.


Letter from Prof. Dr. Roman Rolke
Endowed Chair for Palliative Medicine, Director of the Clinic for Palliative Medicine, RWTH Aachen University Hospital
In Grünenthal’s anniversary year, we can also look back on 22 successful years of the Grünenthal Foundation for Palliative Medicine. Established in December 1998 by Michael Wirtz, the Foundation gained legal status on 8 February 1999 and since then has been dedicated to supporting the first-ever university chair for palliative medicine in Germany, which is at the RWTH Aachen University Hospital.
„We share a common vision.“
On 17 February 2003, Prof. Dr. Lukas Radbruch took on the task of setting up a university palliative medicine department. Today, the RWTH Aachen University Hospital has a 9-bed ward, an outpatient clinic and a palliative medicine service, and it treats up to 1,000 patients each year.
Palliative medicine is a medical treatment approach that is dedicated to providing the best possible quality of life for people who can no longer be cured and are suffering from advanced illnesses, as well as for their relatives. This is done by relieving physical, psychological, social and spiritual problems. The focus is also on treating pain, which is a common mission that connects university palliative medicine and Grünenthal.
The establishment of the Grünenthal Foundation for Palliative Medicine was preceded by personal experiences of the former Grünenthal CEO and current shareholder, Michael Wirtz. In an interview with DIE ZEIT, he described a 1995 trip to Uganda and Rwanda, where he saw refugees and dying people suffering without help in poorly equipped hospitals. These and other experiences moved him to take action in Germany. Alongside other foundations, the creation of the Grünenthal Foundation for Palliative Medicine and the associated chair of palliative medicine were key moments in the development of this field in Germany.
That is why I would like to thank Michael and Michaela Wirtz, who are both supporting the further development of the chair of palliative medicine with great commitment, both personally and with the help of the Grünenthal Foundation for Palliative Medicine.
According to figures from the Hospice and Palliative Care Guide published in August 2019, Germany had 343 palliative care units at that time, and only 68 palliative care services for consultative co-treatment of patients from other hospital departments. There were 243 hospices in Germany, 292 specialised outpatient palliative care (SAPV) services to care for people at home at the end of life, and 1,324 volunteer hospice services. Although these figures, which are now higher, show that Germany is one of the leading nations in Europe in terms of palliative care, there are still major development deficits.
Important steps lie ahead for the organisations and individuals that are active in this field, particularly with regard to research and teaching. In addition to the 11 chairs of palliative medicine in Germany today, further chairs need to be established and top international research needs to be done.
An important cooperation project between university palliative medicine and Grünenthal is a strong example of the way forward in this field. It combines clinical pain research, digital pain recording, PainWatch technologies for biomarker detection, stem cell research, human genetics and artificial intelligence.
Projects like this, as well as other examples, show that Grünenthal is and remains a strong, reliable and innovative partner for pain research.
I warmly congratulate Grünenthal on its 75th anniversary and am grateful that we share a common vision – of a world free of pain!
Yours sincerely,


The year 1946 – Grünenthal’s roots
On 4 January 1946,...
...shortly after World War II, the Stolberg-based entrepreneurial family Wirtz founded a pharmaceutical company under the name “Chemie Grünenthal GmbH”.

This letterhead from 1894 shows the Grünenthal copper yard, which served as a location for the Mäurer + Wirtz soap factory from 1889 to 1913. In 1946, “Chemie Grünenthal GmbH” was founded here.
This letterhead from 1894 shows the Grünenthal copper yard, which served as a location for the Mäurer + Wirtz soap factory from 1889 to 1913. In 1946, “Chemie Grünenthal GmbH” was founded here.
Today...
...in 2021, Grünenthal is a company recognised around the globe as an expert in pain management. We are doing everything we can to drive progress towards our vision of a world free of pain. Grünenthal has its corporate headquarters in Aachen and has affiliates in 29 countries across Europe, Latin America and USA. In 2020, Grünenthal employed around 4,500 people and achieved sales of €1.3 billion.
First success with penicillin
In September 1948...
...Grünenthal marketed its first penicillin products. Just four years later, the family-owned research company, by then collaborating with French and American pharmaceutical companies, was a recognised German antibiotics manufacturer.

The official permission for the production of penicillin, received from the Minister for Economic Affairs of North Rhine-Westphalia in 1948.
The official permission for the production of penicillin, received from the Minister for Economic Affairs of North Rhine-Westphalia in 1948.

The first antibiotic products from Grünenthal at the end of the 1940s.
The first antibiotic products from Grünenthal at the end of the 1940s.
Today...
...Grünenthal is one of the world’s leading companies in the field of innovative pain treatment, selling products in almost 100 countries. In 2018, the company acquired the distribution rights to the products Nexium™ and Vimovo™, and with this acquisition made the largest single investment in Grünenthal’s history.
For a World free of Pain – research at Grünenthal
From the beginning...
...Grünenthal’s management team placed great emphasis on in-house research. A combination of in-house development and cooperation with external partners created a recipe for success when bringing new medicines to the market.


Grünenthal’s research laboratories around 1965.
Grünenthal’s research laboratories around 1965.
In January 2020...
...Grünenthal officially inaugurated its Innovation Hub in Boston, Massachusetts, USA. “The Greater Boston area is known as the largest biotech hub in the world and offers a unique environment for innovation”, says Jan Adams, M.D., CSO Grünenthal.


Pictures from current Grünenthal research facilities. The Boston Innovation Hub is located right at 1 Broadway in Cambridge, Massachusetts.
Pictures from current Grünenthal research facilities. The Boston Innovation Hub is located right at 1 Broadway in Cambridge, Massachusetts.
“All scientists addressing the high medical need in the area of pain management are invited to work with us to develop new therapies for pain patients”.
Medicine for patients – Grünenthal’s global production footprint
Grünenthal began producing antibiotics...
...as early as the 1950s and built a new production facility for them on Zweifaller Strasse in Stolberg. The products from the company’s own research and development activities had to be manufactured and packaged – so the company also placed a strong focus on innovation in production processes from the very start. The multifunctional synthesis of active ingredients in Aachen-Eilendorf, which began operation in 2012, demonstrates the company’s clear commitment to the Aachen production site.
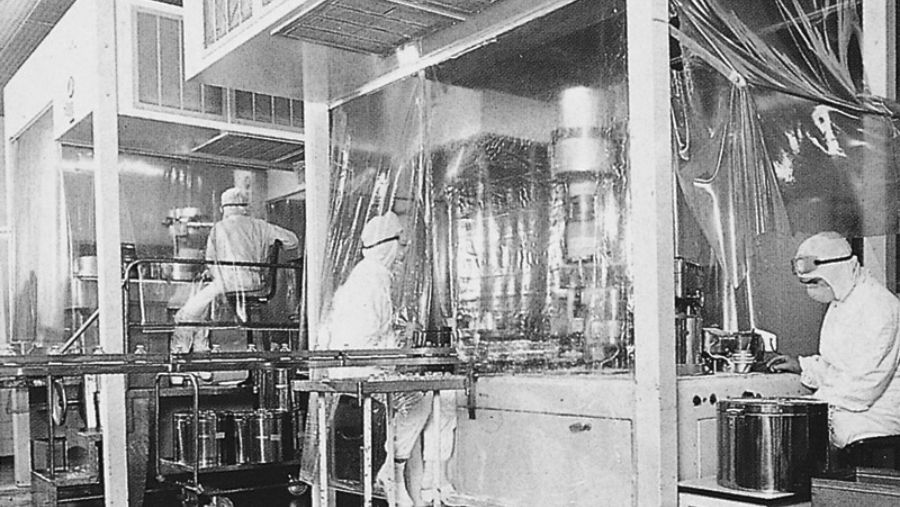

Production in the 1950s.
Production in the 1950s.

The inauguration of the production facility in Ecuador in 1987. From left to right: Bishop Orellana, Dr. Hoff (German Ambassador), Dr. Blasco Penaherrera (Vice President of the Republic of Ecuador), Leon Febres Cordero (President of the Republic of Ecuador), Michael Wirtz, Galo Villamar, Günter Lisken (then Chairman of the Supervisory Board of Grünenthal).
The inauguration of the production facility in Ecuador in 1987. From left to right: Bishop Orellana, Dr. Hoff (German Ambassador), Dr. Blasco Penaherrera (Vice President of the Republic of Ecuador), Leon Febres Cordero (President of the Republic of Ecuador), Michael Wirtz, Galo Villamar, Günter Lisken (then Chairman of the Supervisory Board of Grünenthal).
Grünenthal now operates...
...five production sites in Germany, Switzerland, Italy, Ecuador and Chile. At these locations, products are manufactured and orders from external customers are served, which account for around 50 percent of our total production capacity. Grünenthal’s production facilities are fully integrated, from the production of active ingredients through to packaging and delivery.

The current Grünenthal production facilities in Germany and Switzerland, 2021.
The current Grünenthal production facilities in Germany and Switzerland, 2021.
The Thalidomide tragedy and our responsibility today
Thalidomide was sold as a sedative and sleep aid between 1957 and 1961.
It was marketed by Grünenthal and its licencing and distribution partners under different brand names including Contergan, Softenon and Distaval. What became known in November 1961 was that the drug caused severe deformities in newborn children if it was taken during early pregnancy. The fate of the Thalidomide babies and subsequent court proceedings in Germany are still known today as the “Thalidomide Scandal”.

The patent certificate shows the chemical description of the active ingredient, Thalidomid.
The patent certificate shows the chemical description of the active ingredient, Thalidomid.
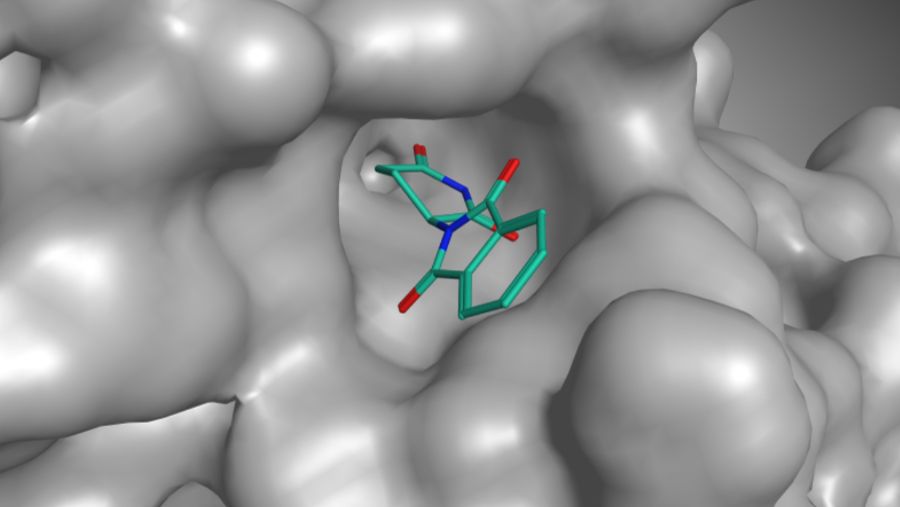
Thalidomide bonds with the protein cereblon, and this can disrupt the healthy development of a child in its mother’s womb.
Thalidomide bonds with the protein cereblon, and this can disrupt the healthy development of a child in its mother’s womb.
The Thalidomide tragedy is and will always remain a significant part of our corporate history.
We take our responsibility for the consequences of the tragedy very seriously. 2021 marks the 60th anniversary of the withdrawal of Thalidomide from the market – a further reason to take another look at how we live up to this responsibility.
We engage in regular dialogue with Thalidomide-affected people and have taken action to express our deep regret for the suffering caused by establishing the Grünenthal Foundation for the Support of Thalidomide-affected People. The Foundation’s mission is to improve the quality of life for people affected by a Thalidomide-containing product from Grünenthal or its licencing and distribution partners by providing specific, needs-oriented support.
For the Foundation’s team, personal communication with affected people is essential. The team takes time to understand the individual needs of those affected and ensures that the Foundation offers support where it is most urgently required. The Foundation supports projects that improve the individual, day-to-day living situation of affected people and expand their opportunities to participate in society. Since it was established, the Foundation has approved more than 2,500 individual applications for support, including providing travel companions or arranging modifications to kitchens and cars. Additionally, the Foundation has implemented various national and international projects.
Further information about the tragedy and the Foundation’s work can be found on the websites www.thalidomide-tragedy.com and www.grunenthal-foundation.com.

The Grünenthal Foundation for the Support of Thalidomide-affected People.
The Grünenthal Foundation for the Support of Thalidomide-affected People.
Building the corporate headquarter
In the years around 1970...
...it became clear that the Grünenthal factory building in Stolberg was too small for the necessary expansion of research and production. In November 1974, Grünenthal acquired a large site in Aachen-Eilendorf from the local government for the purpose of building a modern research centre and raw material production facility. The company’s management team also expected the proximity to the RWTH University Aachen to be advantageous for the company’s own research. The new research centre began operating in the summer of 1977.

The Grünenthal factory building on Zweifaller Straße in Stolberg, around 1970.
The Grünenthal factory building on Zweifaller Straße in Stolberg, around 1970.

Certificate for the laying of the foundation stone at the site in Eilendorf in October 1975.
Certificate for the laying of the foundation stone at the site in Eilendorf in October 1975.
Today...
...the site in Aachen-Eilendorf is home to a modern Grünenthal campus. Alongside the new research building, it features facilities for multifunctional active ingredient synthesis, a warehouse and distribution centre, as well as the packaging centre.
“We have invested approximately €170 million in infrastructure and production in Germany since 2011. This demonstrates our clear commitment to the locations in Aachen and Germany.”
Our apprentices – regularly among the best in class
Education has been an important focus area for Grünenthal since 1948.
With the aim of combining theory and practice, we want to inspire more people to work in this industry. This involves helping young people to gain professional qualifications – and also giving them the opportunity for personal development and responsible project work.

Training in the 1950s.
Training in the 1950s.
In 2020...
...Grünenthal was certified with the “GREAT START!” apprenticeship seal and expanded its range of technical apprenticeships. Today, Grünenthal is one of the largest training companies in the Aachen city region and offers young people individual development opportunities in twelve apprenticeship professions within commercial, scientific and technical fields.

Prof. Dr. Andreas Pinkwart, Minister for Economic Affairs, Innovation, Digitalisation and Energy of the State of North Rhine-Westphalia, during a visit to the training laboratory in 2019.
Prof. Dr. Andreas Pinkwart, Minister for Economic Affairs, Innovation, Digitalisation and Energy of the State of North Rhine-Westphalia, during a visit to the training laboratory in 2019.
The path to becoming an international company
The founding of the first Grünenthal affiliate...
...on the Latin American continent in 1968, Grünenthal Peruana S.A., Lima, was an important step for Grünenthal’s internationalisation. Just four years later, Grünenthal Ecuatoriana C. Ltda. was founded in Quito. Today, Grünenthal is represented worldwide with affiliates in 29 countries across Europe, Latin America and the USA.

Extract from the newsletter of the Asociacion Carl Duisberg del Peru, No. 2, 1993, reporting on the founding of Grünenthal’s first affiliate in Peru in 1968.
Extract from the newsletter of the Asociacion Carl Duisberg del Peru, No. 2, 1993, reporting on the founding of Grünenthal’s first affiliate in Peru in 1968.

Michael Wirtz visits the Peruvian subsidiary in Lima on the occasion of its 35th anniversary in 2003
Michael Wirtz visits the Peruvian subsidiary in Lima on the occasion of its 35th anniversary in 2003

The Grünenthal Tecnandina building in Ecuador in 1987, and today’s office building of the Chilean affiliate.
The Grünenthal Tecnandina building in Ecuador in 1987, and today’s office building of the Chilean affiliate.
By establishing Averitas Pharma...
...a subsidiary of Grünenthal US Holding, Inc. and member of the Grünenthal Group, in 2018, Grünenthal succeeded in entering the US market with a focus on the commercialisation of Qutenza™. In July 2020, Grünenthal achieved an important milestone. Averitas Pharma received approval from the US Food and Drug Administration (FDA) to include the treatment of neuropathic pain associated with diabetic peripheral neuropathy (DPN) of the feet in adults in the US label. A corresponding Supplemental New Drug Application was submitted in the previous year.


Letter from the FDA, dated 17 July 2020, confirming the label extension for Qutenza™.
Letter from the FDA, dated 17 July 2020, confirming the label extension for Qutenza™.
Our products – in-house research success and recent acquisitions
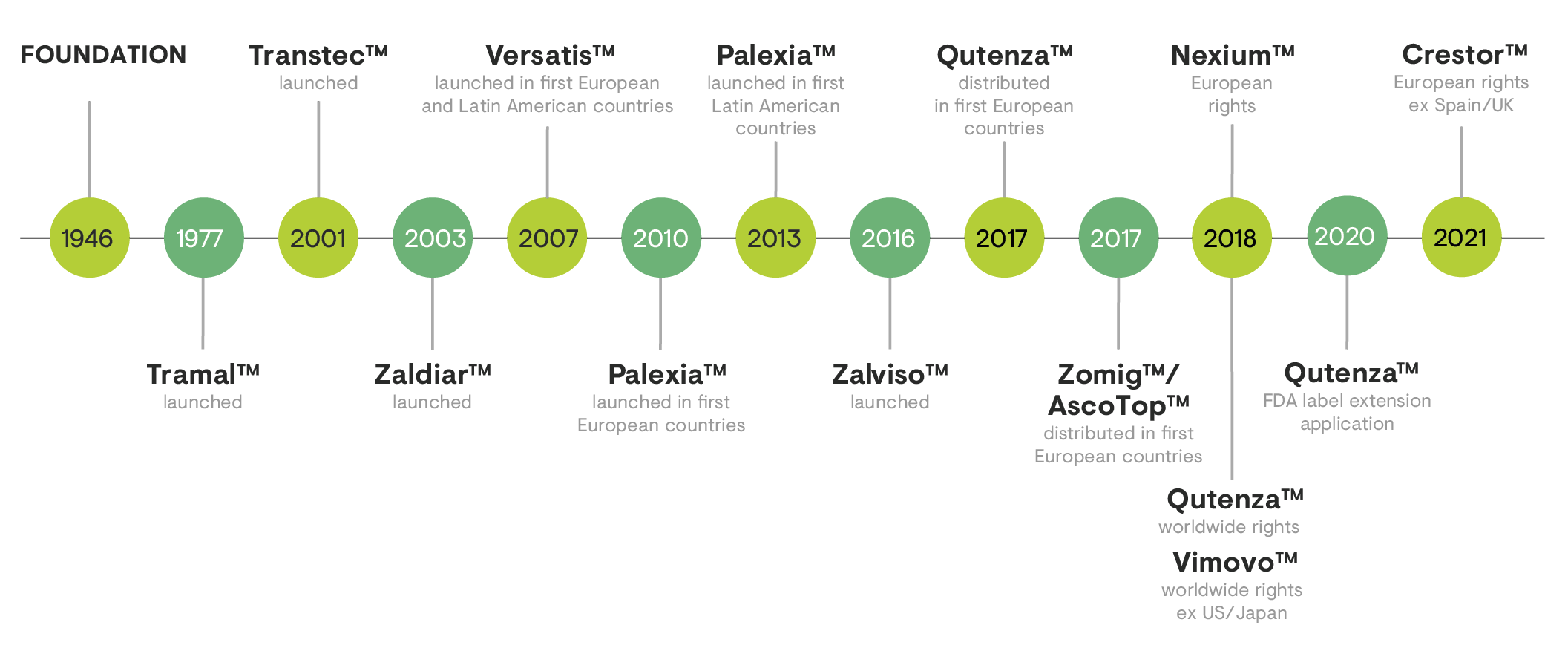
The launch of Tramal™ in 1977 signalled Grünenthal’s transformation into a pain management specialist. In 2001, the European launch of Transtec™ marked another important milestone, providing a safe and easy-to-use treatment for chronic pain. In addition, the launch of Versatis™ in the European Union in 2007 further strengthened Grünenthal’s position as pain expert. However, this journey continues – because there is still need for innovative therapies.

Tramal™
Tramal™
Another milestone in this unique track record for in-house research includes the launch of Palexia™ in 2010, which was the first innovative molecule in the class of centrally acting analgesics (opioids) to be approved in more than 25 years.

Palexia™
Palexia™
One of the largest single investments in the company’s history was the acquisition of the distribution rights for Nexium™ and Vimovo™ in 2018, and for Zomig™/AscoTop™ in 2017.

Qutenza™
Qutenza™
In 2020, Grünenthal achieved another major success, when the U.S. Food and Drug Administration (FDA) approved the supplemental new drug application (sNDA) for the US label of Qutenza™ to now also include the treatment of neuropathic pain associated with diabetic peripheral neuropathy (DPN) of the feet in adults.

Crestor™
Crestor™
The company’s most recent milestones include the acquisition of the European rights (except Spain and the UK) for Crestor™ in 2021, as well as the acquisition of the Swiss company Mestex AG in April 2021, with its innovative investigational medicine RTX (resiniferatoxin) for the intra-articular treatment of pain associated with osteoarthritis of the knee.
Mestex
Mestex
Grünenthal is consistently pursuing its strategy of strengthening and complementing its existing portfolio with well-established brands to continue investing in the transformation of the company and its focused R&D approach.
A strong product portfolio for patients around the world
In our 75-year history, Grünenthal has made great strides towards its vision of a world free of pain – by delivering six pain options to patients. We will keep striving to improve pain management and change the lives of patients around the world for the better”.

An innovative R&D portfolio of promising assets
“I am looking forward to continuing this exciting journey with our team of highly skilled and motivated employees, as well as our external partners. By advancing and complementing our three projects in Phase III and the two projects in Phase I, we will continue to expand Grünenthal’s leadership in pain research and make further progress towards our vision of a world free of pain”.

The Grünenthal Foundation for Palliative Medicine
Palliative and hospice care is a highly relevant social issue.
Michael Wirtz established the Grünenthal Foundation for Palliative Medicine as early as 1998. This was endowed with more than €5 million to cover the running costs of the first-ever university chair for palliative medicine in Germany, which is at the RWTH Aachen University Hospital.

The certificate confirming the creation of the Grünenthal Foundation for Palliative Medicine in December 1998.
The certificate confirming the creation of the Grünenthal Foundation for Palliative Medicine in December 1998.
Today...
...there is a 9-bed ward with an outpatient clinic, as well as a palliative care service. Up to 1,000 patients are treated at the RWTH Aachen University Hospital each year.
Commitment to a better future – initiatives and grants
Our key goal is to provide patients with innovative therapies that improve their quality of life. For this reason, Grünenthal actively participates in a broad range of initiatives and grants that support this important goal.

Grünenthal and the European Pain Federation (EFIC) offer biennial grants for young scientists with a total value of €200,000 to support innovative clinical pain research in each EFIC® member country. The joint initiative has been in place since 2004.
www.e-g-g.info

Change Pain™ is an initiative for the treatment of chronic pain that was launched by Grünenthal in 2009. It is supported by the European Pain Federation (EFIC), the Pain Alliance Europe (PAE), and the European Society of Regional Anaesthesia and Pain Management (ESRA). The CHANGE PAIN™ medical education platform is a global communication centre that provides healthcare professionals and patients with tailored educational content about pain.
www.change-pain.com

Grünenthal is one of the main sponsors of Societal Impact of Pain (SIP), a multi-stakeholder partnership that is supported by the European Pain Federation (EFIC) and the Pain Alliance Europe (PAE). Since 2009, Grünenthal has been working to highlight the relevance of the impact that pain has on societies, healthcare systems and economic systems – and to reduce this impact.
www.sip-platform.eu

Grünenthal provides financial support for the Brain, Mind and Pain (BMP) GRANT, which promotes patient-centred innovation in pain research and pain management. The BMP GRANT is the first pan-European grant that funds applications on the basis of the impact that they have from the patient’s perspective.
www.bmp-grant.eu
Our path to the future

Grünenthal is well positioned for the future. Thanks to the tireless efforts of our employees, we were able to ensure an uninterrupted supply of important medicines to patients during the Covid-19 pandemic. Our established brands and acquisitions will continue to drive growth and strengthen our sales. Our entry into the bond market in the spring of 2021 will also give us the necessary financial flexibility to invest in research and development for important therapies in the area of pain.
To mark our 75-year anniversary, 7,500 trees will be planted around the world during the year. This is a symbol of our commitment to People, Patient and Planet – three areas that summarise the flagship initiatives of our Corporate Responsibility Programme. Responsibility and sustainability are central elements of our strategy at Grünenthal.
Our journey towards a World free of Pain

Back row, from left to right: Fabian Raschke, Chief Financial Officer (CFO), Member of the Corporate Executive Board; Gabriel Baertschi, Chief Executive Officer (CEO), Chairman of the Corporate Executive Board; Quentin Le Masne de Chermont, Head Corporate Strategy, Member of the Executive Board Team; Victor Barbosa, Head Global Operations, Member of the Executive Board Team; Mark Fladrich, Chief Commercial Officer (CCO), Member of the Corporate Executive Board. Front row, from left to right: Jan Adams, M.D., Chief Scientific Officer (CSO), Member of the Corporate Executive Board; Sebastian Köhler, General Counsel, Member of the Executive Board Team; Leen Hofkens, Head Global Human Resources, Member of the Executive Board Team.
Back row, from left to right: Fabian Raschke, Chief Financial Officer (CFO), Member of the Corporate Executive Board; Gabriel Baertschi, Chief Executive Officer (CEO), Chairman of the Corporate Executive Board; Quentin Le Masne de Chermont, Head Corporate Strategy, Member of the Executive Board Team; Victor Barbosa, Head Global Operations, Member of the Executive Board Team; Mark Fladrich, Chief Commercial Officer (CCO), Member of the Corporate Executive Board. Front row, from left to right: Jan Adams, M.D., Chief Scientific Officer (CSO), Member of the Corporate Executive Board; Sebastian Köhler, General Counsel, Member of the Executive Board Team; Leen Hofkens, Head Global Human Resources, Member of the Executive Board Team.

Grünenthal logos through the ages.
Grünenthal logos through the ages.
Think Innovation. Feel Life.