Our Vision:
A World Free of Pain

DEAR READERS,
At Grünenthal, we're dedicated to transforming the world of pain management by seeking out innovative treatment options to change lives for the better.
Over the last years, we have transformed the company, diversified our portfolio, and strengthened our innovation pipeline. We are now in a prime position to continue our growth strategy and move closer to our vision of a World Free of Pain. We achieved major milestones while exceeding our financial and non-financial targets.
Learn more about our journey, our strategy moving forward, important projects and the people behind it all. Together with this great team, I am proud to work for a World Free of Pain.

Gabriel Baertschi
Chief Executive Officer


DEAR READERS,
At Grünenthal, we're dedicated to transforming the world of pain management by seeking out innovative treatment options to change lives for the better.
Over the last years, we have transformed the company, diversified our portfolio, and strengthened our innovation pipeline. We are now in a prime position to continue our growth strategy and move closer to our vision of a World Free of Pain. We achieved major milestones while exceeding our financial and non-financial targets.
Learn more about our journey, our strategy moving forward, important projects and the people behind it all. Together with this great team, I am proud to work for a World Free of Pain.

Gabriel Baertschi
Chief Executive Officer
1 in 5 people
suffer from chronic pain worldwide.1


Almost everyone knows different types of pain from personal experience. In most cases, pain improves over time and passes eventually. For many people worldwide, however, pain is an everyday, constant reality. Pain is one of the most common reasons for seeking medical help.
In chronic pain syndromes, pain can be the sole or a leading complaint or can be secondary to an underlying disease.2 If it lasts longer than three months, it is referred to as chronic pain.3
Chronic pain is a significant burden for patients, their families, caregivers, and society: It is a frequent cause of people withdrawing from the labour market early and a significant contributor to disability retirement.4 Its alleviation remains an important unmet medical need, as many patients struggle to find effective relief from available medicines.
Leading the way towards a World Free of Pain

Shaping the future of pain management
Grünenthal is the leading pharmaceutical company focused on pain therapies and research. We have dedicated the last 50 years to delivering innovative treatment options for people affected by pain. Now, we accelerate our push toward achieving our vision of a World Free of Pain. We are committed to transforming the future of pain management by developing the next generation of pain medicines.
We have a strong corporate strategy for achieving our vision of a World Free of Pain: We seek to relieve pain and make life better for patients and their families with every research project we launch and every medicine we create. Looking back, we feel proud of our achievements in further addressing the unmet need of pain patients.
Our business results prove that we are heading in the right direction.
Our Research & Development (R&D) activities focus on four indications that affect many individuals worldwide:

Peripheral neuropathic pain

Chronic low back pain

Osteoarthritis

Chronic post-surgical pain
Another year of exceptional performance
Fuelled by a successful transformation and growth strategy, Grünenthal exceeded its financial and non-financial targets in 2022.
Net revenues reached €1.7 billion, an increase of 13 percent compared to 2021. The adjusted EBITDA reached €438 million, an increase of 18 percent over 2021.
Our record results can be attributed to increased demand for pain treatments and excellent sales performance across regions: All key brands performed well and grew faster than the market.

Revenue from pain products accounted for 59 percent of total revenues in 2022 which underlines our positioning as the leader in pain management. In addition, the split of revenue by geography indicates global diversification, enabling us to manage business risks more effectively and making us less dependent on a single product or market.
The acquisition of carefully selected established brands has been instrumental in our profitability and growth. Grünenthal acquired Nebido™ from Bayer for approx. €495 million. The brand immediately contributed to Grünenthal’s revenue and profit as of November 2022. Another milestone in Grünenthal’s Merger & Acquisition strategy (M&A strategy) was the announcement to enter into a joint venture agreement with Kyowa Kirin International. The joint venture includes 13 established brands with revenues primarily from pain management products.

Revenue and profit contributions from these acquisitions help secure our financial stability and enable us to reinvest in pain research, thereby advancing our R&D pipeline and moving towards our vision of a World Free of Pain.



Basis for further growth

Over the past few years, Grünenthal has fundamentally transformed its strategy and culture, laying the foundation for achieving our shared vision of a World Free of Pain, which we launched in 2017.
Our exceptional results prove our transformation efforts are bearing fruit. Since 2017, Grünenthal’s profitability measured by adjusted EBITDA, has more than tripled, in terms of equity market value and operating cash flow. During the same time, we have entered the debt capital market and have received strong credit ratings from major independent rating agencies.
Our researchers have dramatically expanded our innovation pipeline, which now encompasses three Phase III projects, two Phase I projects and innovative pre-clinical platforms. This reflects the success of our R&D strategy, which has created a modern operating model that enables our scientists to pursue high-potential assets in a modality-agnostic manner. Since adopting this setup in 2019, we have become more diverse and international. Also, we pursue a global approach that includes partnerships with organisations that share our passion for scientific progress.
We also continue to see strong commercial performance across our portfolio and geographies.
QutenzaTM, a non-opioid treatment for various neuropathic pain conditions, has become an even more powerful potential growth driver following a US label extension in 2020, and a significant ramp-up of our US commercial infrastructure and promotional activities in 2021/22.
Another case in point towards the success of Grünenthal’s transformation is the performance of Latin American markets where one third of Grünenthal’s revenues are being generated. Since 2017, we have focused on innovative pain products (+30% growth) and profitable diversified products (+23% growth), which led to an increased profitability of the region.
Global Operations (GO) was established as a new global business area enabling end-to-end processes for our product supply worldwide in 2017. At the same time, GO has increased efficiency by streamlining the organisation and its processes and enabled external growth by integrating new products into our supply chain quickly and effectively. Our operations teams always ensured reliable supply to patients despite major external distress.
Striving for a World Free of Pain

Grünenthal is dedicated to developing and commercialising innovative pain products.
We aim to alleviate or eliminate pain for patients who are still seriously underserved in this therapeutic area. Our vision of a World Free of Pain is built on five pillars: innovation, growth, acquisitions, efficiency, and people. All five elements are essential and closely linked.
The 5 pillars of our corporate strategy

Innovation
Be a leading innovator in pain treatments to address critical, unmet medical needs, with a focus on non-opioid treatments.

Growth
Drive the commercial success of our growth brands and evolve our go-to-market model towards digital and omnichannel approaches.

Acquisitions
Complement our portfolio with deals for established brands, irrespective of therapeutic area.

Efficiency
Drive profitability through efficiencies across the value chain and manufacture at the best safety, quality, and cost level.

People
Invest in building the capabilities of our people and operate in line with the highest ethical and regulatory standards.
Innovation for next-generation pain medicines
Existing pain therapies work for some patients – but not for all of them. One European survey revealed that 40 percent of patients were unsatisfied with their pain management.6
This shows the clear need for innovative treatment options that provide better outcomes for more patients.
It also motivates us to engage in pioneering research to find next-generation, non-opioid pain medicines and develop advanced technologies to bring life-changing treatments to patients in need.

Our deep understanding of the underlying human disease biology is at the core of our R&D efforts: our researchers work to understand the role each target performs when processing pain in the body. Targets are biological structures in our body on which a potential new drug can act to inhibit pain transmission. By combining our research of the target’s function with existing clinical and genetic evidence, we identify well validated, highly promising targets that may become the basis for new therapeutic options.
To tap into the best science and technologies, we collaborate with leading institutions worldwide.
With our partners at Uniklinik RWTH Aachen and RWTH Aachen University, for example, we work on solving challenges related to models for investigating new pain medicines. Until now, scientists have used rodent behavioural models to explore the cellular and molecular mechanisms involved in pain. However, there are fundamental differences in pain mechanisms across species.
Together, we strive to increase our ability to validate potential new treatments using human cells and tissue during pre-clinical testing. This will involve creating a shared local infrastructure to ethically and reliably source human Dorsal Root Ganglia (DRGs) and other tissues of interest. DRGs are key gatekeepers that decide which signals are forwarded to the central nervous system and, ultimately, the brain, where one perceives these signals as pain. If successful, the results of this cooperation will enable new methods to be integrated into drug development activities at Grünenthal.
Our cooperation with King’s College London aims at further expanding our pre-clinical toolbox. We are developing microfluidic culture (MFC) models that investigate neurons derived from human induced pluripotent stem cells (iPSC). MFCs can more accurately represent a cell’s natural micro-environment and in-vivo milieu than traditional in-vitro models. Combined with iPSC derived neurons that very closely mimic the functionality of human nociceptive neurons, which are responsible for pain transmission, we may significantly enhance our understanding of how investigational medicines modulate pain in humans.

Pain Research at Grünenthal

Focused therapeutic area strategy
We focus our R&D efforts on four pain indications characterised by high unmet medical need.

Comprehensive disease understanding
Deep understanding of the underlying human disease biology enables us to identify well validated, highly promising targets.

Double down on most promising targets
We pursue targets holistically and leverage a wide range of modalities to minimise compound-specific risks and maximise probability of success.

Teaming up
We collaborate with leading institutions around the world to tap into the best science and technologies wherever they exist.
Researching a novel treatment option for osteoarthritis
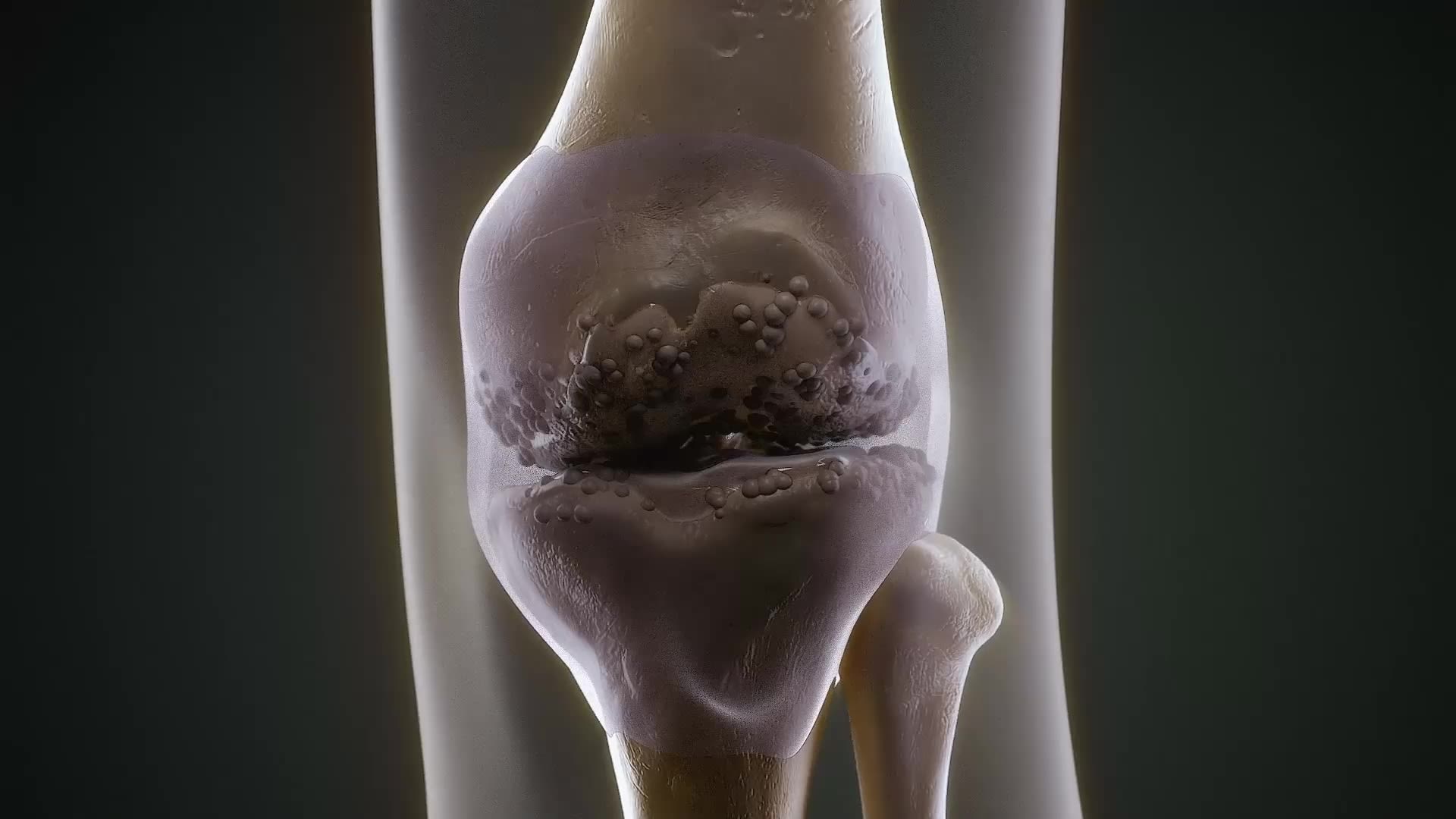
Osteoarthritis is one of our R&D focus areas. This progressive condition is the most common joint disease in people 65 years of age and older, mainly affecting the knees, hands, hips, neck, and lower back.
Osteoarthritis causes tissue in the joints to break down over time. Currently, there is no cure for this condition. Patients suffer from inflammation, pain, and stiffness.
Our experts aim to provide a non-opioid therapy option for patients affected by pain associated with osteoarthritis of the knee. This condition affects over 360 million people worldwide.7 Patients with knee osteoarthritis typically receive a range of anti-inflammatory medicines, many will also receive intra-articular corticosteroids or even undergo knee replacement surgery to relieve the pain of inflamed and swollen joints.
Resiniferatoxin is a non-opioid intra-articular treatment that has the potential to provide long-lasting pain relief and functional improvement of the joints. RTX is a highly potent Transient Receptor Potential Vanilloid 1 (TRPV1) agonist developed based on research that won the Nobel Prize in Physiology or Medicine in 2021.
Having secured the global rights for RTX through a 2021 acquisition, our experts are making constant progress to bring this potential life-changer closer to a market launch.
Just one year after the acquisition, the Grünenthal team enrolled the first patients in the global clinical Phase III programme for RTX. The programme will include more than 1,800 patients with knee osteoarthritis who have exhausted available treatment options and still suffer from moderate to severe pain.
With this programme, Grünenthal is now investigating the efficacy and safety of intra-articular injections of RTX in adults by conducting three trials across approximately 200 sites in Europe, the US, Latin America, South Africa, and Japan. The Phase III programme may enable marketing approval for RTX in the EU, the US, and Japan and other countries worldwide, if successful.
Our partnership with the US-based life science investment firm NovaQuest Capital Management supports these Phase III trials. The agreement signed in 2022 significantly contributes to securing the development costs for RTX. This was the first time that Grünenthal has agreed a strategic collaboration of this type.
Grünenthal is also engaging in partnerships that aim to maximise the geographical footprint of RTX beyond Grünenthal’s core markets in the EU, the US, and Latin America. In August 2022, we entered a licensing agreement with Shionogi & Co., Ltd. a leading global research-driven pharmaceutical company based in Japan. With this deal, Shionogi obtained exclusive rights to commercialise RTX in Japan.
If the drug is approved by regulators, we envisage a potential market entry for RTX in 2025/2026.
Our company holds the global rights for this treatment – and the global osteoarthritis market is expected to grow from $7.3 billion in 2020 to approximately $11.0 billion in 2025.8 Grünenthal intends to explore the potential of RTX for treating osteoarthritis-related pain in additional joints if the outcome of the Phase III programme is positive.
Maximising the potential
of our portfolio
Grünenthal’s portfolio includes ten global brands at different stages of the brand lifecycle.
Due to its complementary mix of established and growth brands, Grünenthal is well-positioned to maximise business opportunities and build successful brands now and in the future.
In 2022, revenue from growth and established brands increased significantly, leading to our company’s best financial performance. Combining these two product categories gives us a well-balanced and resilient business and basis for further growth.
The established brands bring together all mature and off-patent products. They are characterised by high brand awareness, predictable and stable sales, and high profitability. They include Nexium™, Crestor™ and Nebido™, and brands we have developed over a longer period, like Tramal™. We continue to proactively manage these brands through a customer-centric approach delivered via a range of channels. Our differentiated strategies also reflect the specific market conditions for each treatment.
Our established brands achieved €1,047 million in 2022, which is 69% of Grünenthal’s operational revenue. Almost all established brands grew faster than the market and delivered increased year-on-year sales.

© Grünenthal
© Grünenthal
In 2022, our growth brands comprised innovative and patent-protected products like Qutenza™, and brands that continue to have valuable growth potential like Palexia™ and Vimovo™.
Qutenza™ is a topical system containing prescription-strength capsaicin. It is a non-opioid treatment that can provide prolonged pain relief for several months, while most frequently reported adverse events were transient, self-limiting, mild to moderate application site reactions.9
In Europe, it is approved for the treatment of peripheral neuropathic pain. In the US, it is approved for the treatment of peripheral neuropathic pain associated with post-herpetic neuralgia. In 2020, it also received approval for treating pain associated with diabetic peripheral neuropathy (DPN) of the feet in adults.10 Painful DPN is a progressive and debilitating complication of diabetes that affected more than five million Americans in 2020.11 The label extension marked a significant milestone in making Qutenza™ more widely available for patients in need – and we continue these efforts particularly in the US.
We have further invested in building inspiring medical education and a patient support programme focused on the best use of Qutenza™. Our peer-to-peer education programme aims to better communicate the science of Qutenza™, hence improving the quality of patient care.
For Qutenza™, demand from healthcare professionals rose during 2022 with over 77,000 patients treated globally – 16 percent more than in the previous year – with strong growth in all countries where Qutenza™ is on the market. Its global sales amounted to €76 million in 2022 (+ €33 million, + 77%; without license and milestone income).



Palexia™, a treatment option for patients with severe chronic pain where a doctor has decided that an opioid is necessary, is still growing after more than a decade on the market due to its differentiated profile. In 2022, its global sales amounted to €333.7 million (+ €17 million, + 5%; without license and milestone income). Building on new real-world evidence data, the Palexia™ team has been working on helping physicians better understand how Palexia™ creates value for patients where a doctor has decided that an opioid is necessary. At the same time, we are preparing for and will manage the upcoming loss of exclusivity of Palexia™ in Europe.
We have a continuing commitment to explore and endorse measures that minimize the risk of inappropriate and illegitimate use of prescription opioids – while striving to ensure that individual patients with a clear need for opioid-based pain relief are not denied access. Please see our statement regarding the responsible use of opioid-based medicines.
Across all brands, we have continued to evolve our go-to-market model towards a digital and omnichannel approach. This has been accelerated by the Covid-19 pandemic, and we have rapidly and substantially expanded our use of channels that enable remote interaction.
Leveraging strategic acquisitions

While we continue to develop our existing brands and grow organically, we also tap into inorganic growth opportunities by acquiring assets that strengthen our established brand portfolio – no matter which therapeutic area.
Since 2017, Grünenthal has closed successful acquisitions with a total expected deal value of more than €2.0 billion.
Grünenthal acquired Nebido™, a leading brand for symptomatic male hypogonadism, when testosterone deficiency has been confirmed by clinical features and biochemical tests, from Bayer for approx. €495 million. The brand immediately contributed to Grünenthal’s revenue and profit as of November 2022. Some Grünenthal acquisitions are also made through collaborations and joint venture arrangements, like our latest deal with Kyowa Kirin International, a Japan-based global specialty pharmaceutical company. The joint venture agreement includes 13 established brands with revenues primarily from pain management products.
Our deals deliver significant EBITDA contributions that enable us to continue investing in research and development of innovative pain treatments. Zomig™, Nexium™ and Vimovo™, Crestor™ as well as Nebido™ - all acquired since 2017 - contributed €278 million to Grünenthal’s adjusted EBITDA, representing 63 percent of our total adjusted EBITDA for 2022.
These acquisitions and collaborations will further contribute to our solid EBITDA growth in 2023. In addition to this, synergies play an important role after the integration of acquired brands. In 2022, manufacturing synergies for Nexium™, Vimovo™ and Zomig™ reached around €12 million.
Excelling in production

It is Grünenthal’s experts in Global Operations (GO) who manage the integration of new products and technologies into our company quickly and effectively, thereby maximising the return on investment.
Here, the GO team is building on its expertise in driving profitability by identifying efficiencies across the value chain for all brands at every stage in the product lifecycle.
In ensuring the highest levels of safety, quality and cost-efficiency in our manufacturing activities, our GO team strives to ensure patients have reliable access to medicines across more than 100 countries worldwide. We are proud that our manufacturing and operations teams successfully kept our business running at all times – despite the pandemic, global supply challenges and disrupted supply chains.

© Grünenthal
© Grünenthal
Around 2,000 people manage the full end-to-end value chain for our products. We operate five specialised production facilities – in Chile, Ecuador, Germany, Italy, and Switzerland – which we continue to develop to maintain our strong competitive position. Between 2020 and the end of 2023, we will have invested more than €140 million.
At our sites, we manufacture Grünenthal products, and we also support external customers as part of our Contract Manufacturing Business, Grünenthal PRO.
Global Operations reached an overall increase in production volume in 2022. For 2023, we expect to further increase our overall production volume by 13 percent compared to 2022. This growth is driven by the successes of our brands and our global Partner Business expansion – and reaches across all our global sites.



Making use of digitalisation

We take advantage of smart innovations inspired by Industry 4.0 to maximise productivity, improve our reactions to market changes and make our manufacturing processes more resilient.
These technologies include data capture, advanced analytics, and assembly line robotics. Our focus is on strengthening our fundamentals by creating more efficient processes and enabling smoother end-to-end operations:
- In packaging, we have introduced cobots for highly repetitive activities like carton handling. In the warehouse, we use Autonomous Guided Vehicles (AGVs) to increase efficiency, reduce errors and improve safety.
- We have also introduced new systems for data collection, as well as data and relationship management, such as an automated and standardised digital performance system across all sites or a new eProcurement platform for tendering, offer comparison and supplier relationship management.
- In addition, we have implemented the E2Open platform to connect to our Enterprise Resource Planning (ERP) systems allowing for automatic exchange of supply chain data, such as information about orders, updates, inventory levels and deliveries. This platform takes our digitalisation to the next level, increases transparency, and improves our process management.
Joining forces for impact






Grünenthal’s exceptional performance is a testament to our more than 4,000 people and their dedication towards achieving our vision of a World Free of Pain.
At Grünenthal, we join forces to contribute to this vision – and we strongly believe that creating an environment where all employees feel valued, respected, and involved, and can bring their full selves to work, empowers all of us to contribute best.
In 2022, we launched our Diversity and Engagement Strategy, reflecting our priorities: enhancing diversity, driving conscious inclusion, and positively impacting our local communities. It provides specific global commitments and will enable us to achieve outstanding business results and build the capabilities we need for the future.
Currently, Grünenthal employs individuals from 28 countries, holding 63 different nationalities. Our gender ratio is balanced across all employees; the share of women has increased in both leadership and executive roles compared to 2018.
Across our organisation, our employees continue to provide positive feedback about our workplace culture and leadership approach. As part of our feedback system, we have been conducting the Great Place to Work® survey since 2009. In 2022, we were certified as a Great Place to Work® in 24 entities spread across 19 countries. Looking ahead, we will maintain our drive towards a high-performance culture while continuing to strengthen opportunities for personal and professional development. This also includes our activities to fuel our future talent pipeline by offering graduate and trainee programmes that provide a smooth and varied start to professional life.
Grünenthal aspires to create a positive impact on society – in our core business and beyond.
Our ambitions drive our approach to Corporate Responsibility and our firm commitment to integrity, transparency, and the highest ethical standards always guides us. This encompasses our role as an advocate for the responsible use of our products – including medically necessary opioids. To ensure highly effective compliance processes, we have instilled a culture that gives our company an ethically minded and fully engaged workforce. It also drives us to maximise its beneficial effect on employees, partners, and society – while reducing the environmental footprint of our business activities.
Our performance against these Environmental, Social, and Governance (ESG) criteria is recognised by excellent external ratings. In 2022, Grünenthal’s second ESG rating confirmed the company’s improved ESG risk management, putting it in the top three percent of the global pharmaceutical subindustry. We strive to continuously improve and optimise our performance to live up to our stakeholders’ and our own expectations.

© Grünenthal
© Grünenthal


© Grünenthal
© Grünenthal

© Grünenthal
© Grünenthal

© Grünenthal
© Grünenthal

© Grünenthal
© Grünenthal

© Grünenthal
© Grünenthal

ABOUT GRÜNENTHAL
Grünenthal is a global leader in pain management and related diseases. We have a long track record of bringing innovative treatments to patients worldwide.
As a fully integrated pharmaceutical company we cover the entire value chain – from drug research and development to commercialisation of portfolios with both growth products and established brands. We operate in accordance with the highest ethical and regulatory standards, and we focus our efforts on our vision of a World Free of Pain.
We are a global company headquartered in Aachen, Germany, with 5 manufacturing sites in Europe and Latin America as well as an international R&D network with sites in Germany and the United States. Currently, we have affiliates in 28 countries across Europe, Latin America, and the US, and some 4,400 employees worldwide.
Grünenthal now touches the lives of millions of patients every year in around 100 countries, with innovative treatments that reduce suffering and give patients the quality of life they deserve.
If you are interested in learning more about Grünenthal as a company, its vision of a World Free of Pain and how it aims to achieve this vision, please visit www.grunenthal.com or follow us on LinkedIn.
Access the detailed PDF of the Annual Report or our latest Corporate Responsibility Report.

References
1. TreedeRD, et al. Pain. 2015 Jun;156(6):1003-1007
2. TreedeRD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, GiamberardinoMA, Kaasa S, KorwisiB, Kosek E, Lavand'hommeP, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, VlaeyenJWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019 Jan;160(1):19-27.)
3. World Health Organization (WHO). International Classification of Diseases 11th Revision (ICD-11). MG30 Chronic pain. 2019.
4. Saastamoinen P, Laaksonen M, KääriäSM, LallukkaT, Leino-Arjas P, Rahkonen O, Lahelma E. Pain and disability retirement: a prospective cohort study. Pain. 2012 Mar;153(3):526-531.
5 Pain Alliance Europe. Survey on Chronic Pain. 2017: Diagnosis, Treatment and Impact of Pain. 2017. Available at: https://www.pae-eu.eu/wp-content/uploads/2017/12/PAE-Survey-on-Chronic-Pain-June-2017.pdf (Accessed April 2023)
6. BreivikH, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. EurJ Pain. 2006;10(4):287-333
7. Cieza, A., Causey, K., Kamenov, K., Hanson, S. W., Chatterji, S., & Vos, T. (2020). Global estimates of the need for rehabilitation based on the Global Burdenof Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet, 396(10267), 2006-2017
8. Markets And Markets Report. (2020). Osteoarthritis Therapeutics Market by Anatomy (Knee, Hand), Drug Type (NSAIDs, Analgesics, Corticosteroids), Route of Administration (Parenteral), Distribution Channel (Hospital Pharmacies), Purchasing Pattern (Prescription Drugs) -Global Forecast to 2025. Available at: https://www.marketsandmarkets.com/Market-Reports/osteoarthritis-therapeutics-market-209565994.html (Accessed November 2022)
9. Summary of Product Characteristics (SmPC) Qutenza™
https://www.grunenthalhealth.com/-/media/projects/producthub/shared/basic-product-pages/english-hq/qutenza/qutenza-epar-product-information-en-smpc.pdf?rev=268de8dafbca4f258f8ca4f5598b05eb (Accessed May 2023)
10. Qutenza™ [prescribing information]. Morristown, NJ: AveritasPharma https://www.qutenza.com/pdfs/Qutenza_Prescribing_Information.pdf (Accessed May 2023)
11. LTP 2020-2030: ADDRESSABLE POPULATIONS BY CONDITION (PDPN, PSNP, PHN, CINP). Company data on file. April 30, 2020
IMPRINT
Contact
Florian Dieckmann
Head Global Communication
E-mail: GlobalCommunication@grunenthal.com
Publisher
GRÜNENTHAL PHARMA GMBH & CO. KG
Zieglerstrasse 6
52078 Aachen
Germany
Phone: +49 241 569 0
E-mail: info@grunenthal.com
www.grunenthal.com
Photo Credits: Grünenthal, Image Professionals GmbH, Petra Eich, Adobe Stock, Getty Images
Disclaimer
Privacy


