Pain has a huge and growing impact on patients, families, communities and healthcare systems around the globe.1
people suffer from chronic pain worldwide.2
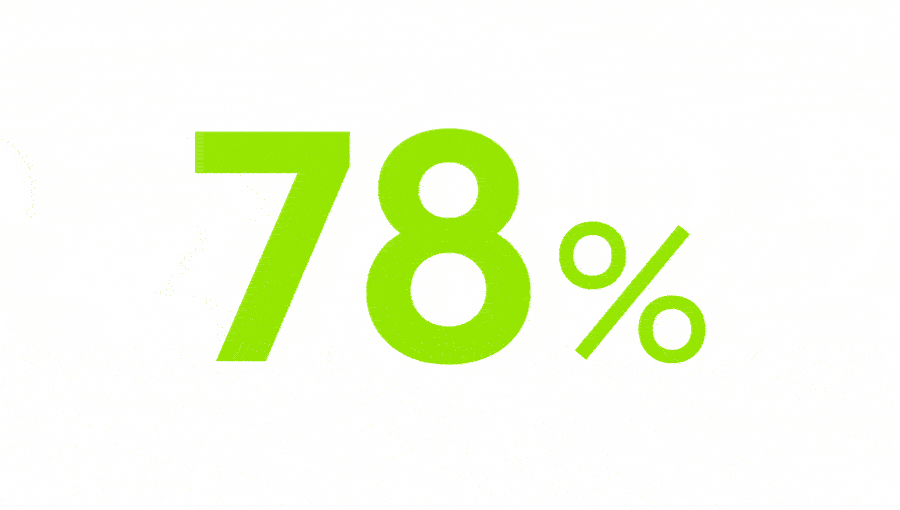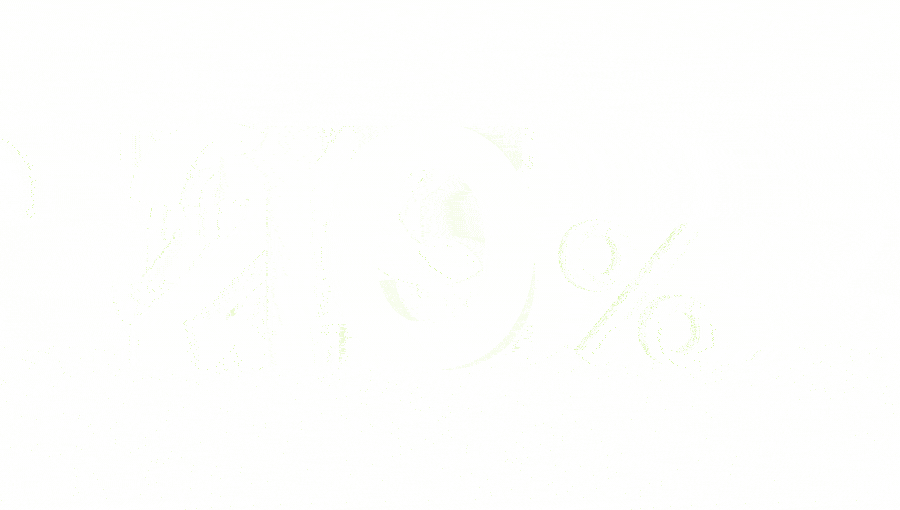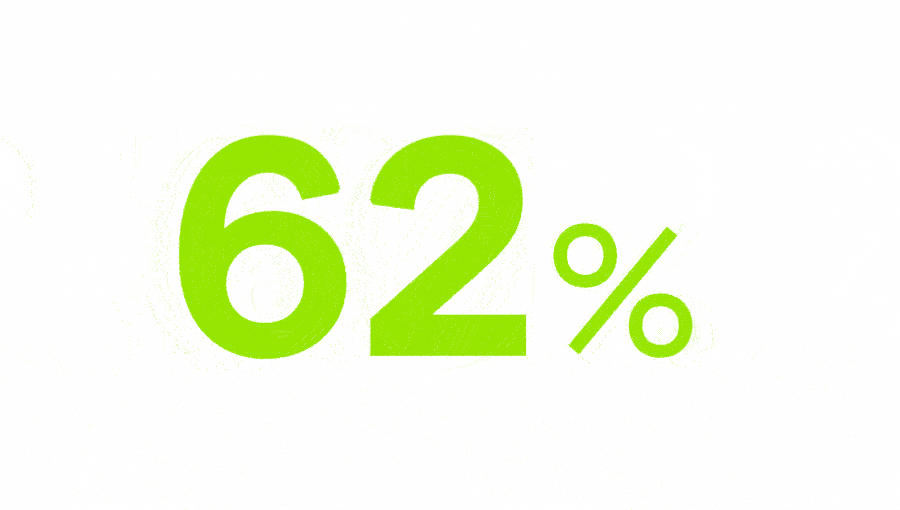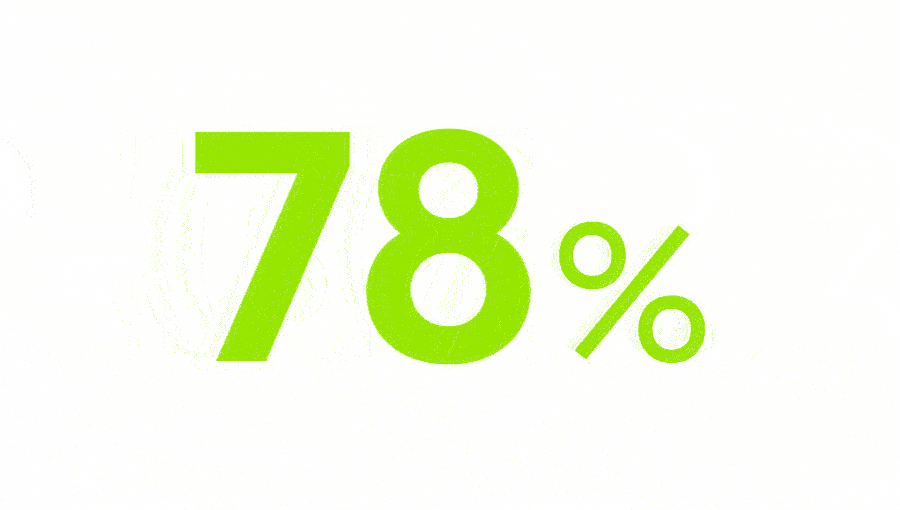
of chronic pain patients in Europe are not satisfied with their treatment.3
is the estimated cost of chronic pain across Europe.3
We as pain expert
Grünenthal is the leading pharmaceutical company with a clear focus on pain therapies and research.
We have dedicated the last 50 years to delivering innovative pain medicines for patients with unmet needs.
Now, we are pursuing our vision of a World Free of Pain by developing next-generation treatments that make life better for pain patients and their families.

Our Company.
Leadership, Transformation, Strategy, Product Portfolio & Finance

LEADERSHIP TEAM
Gabriel Baertschi
Chief Executive Officer
Read more
Jan Adams, MD
Chief Commercial Officer
Read more
Fabian Raschke
Chief Financial Officer
Read more
Uli Brödl, MD
Head of Research & Development, Chief Scientific Officer
Read more
Victor Barbosa
Head Global Operations
Read more
Leen Hofkens
Head Global Human Resources
Read more
Sebastian Köhler
General Counsel
Read more
Quentin Le Masne de Chermont
Head Corporate Strategy and Portfolio Management
Read more
Gabriel Baertschi
Chief Executive Officer
Gabriel joined Grünenthal in 2016 as Chairman of the Corporate Executive Board and CEO. As a biologist, his love of science and dedication to improving patients’ lives led him to work for the pharma industry. His strong leadership and clear vision have enabled Grünenthal to transform its business. He has executed a diligent strategy with EBITDA-accretive acquisitions, a promising R&D pipeline and strong financial performance that has tripled the company value since 2017. Gabriel is a non-executive board member at DKSH, a Swiss stock-listed company where he serves on the Compensation and Remuneration and the M&A committees. He is a non-executive board member at MedXCell, a Swiss biotech company.
Jan Adams, MD
Chief Commercial Officer
Jan assumed the role of Chief Commercial Officer and Member of the Corporate Executive Board in October 2024. Since 2019, he had led Grünenthal's R&D organisation as Chief Scientific Officer and Member of the Corporate Executive Board. He has over two decades of experience in the pharmaceutical and healthcare industry. Since joining Grünenthal in 2017, he has played a key role in driving the company's transformational journey. Under his leadership, Grünenthal successfully built a state-of-the-art R&D organisation, redefined its R&D strategy, and built an industry-leading pipeline focused on delivering innovative treatments for acute and chronic pain. Before assuming the role of CSO, he served as Head of Strategy and Portfolio, working at the interface between Strategy, R&D and Commercial. His role was instrumental in several successful M&A projects and Grünenthal’s entry into the US market.
Uli Brödl, MD
Head of Research & Development,
Chief Scientific Officer
Through more than 15 years in the pharmaceutical industry, Uli oversaw end-to-end clinical development and operations activities across therapeutic areas and worked at the interface of R&D, Commercial, and Market Access functions. He has a proven track record of bringing innovative medicines to patients, including Empagliflozin, an oral anti-diabetic medicine.
Uli joined Grünenthal in 2025 from Boehringer Ingelheim, where he served as Corporate Senior Vice President, Head of Global Clinical Development & Operations, and member of Boehringer Ingelheim's Venture Fund Investment Committee. At Grünenthal, he took over the role of Chief Scientific Officer and assumed leadership of Grünenthal's R&D organisation.
Fabian Raschke
Chief Financial Officer
In his career, Fabian has a proven track record of success in projects ranging from completely modernising a company’s Finance function to increasing efficiency, driving growth and taking advantage of the full range of financing models. Fabian joined Grünenthal in 2016 as Head Group Controlling, before assuming the role of Chief Financial Officer in 2019. He was pivotal in Grünenthal’s move to the capital markets with the first bond placement in 2021. Fabian is also responsible for the realignment of the IT function, supporting our digital roadmap and substantially increasing our cyber defence capabilities.
Victor Barbosa
Head Global Operations
Since joining Grünenthal in 2006, Victor has worked across the organisation’s supply chain and operations teams. With extensive experience in diverse markets, he has been instrumental in redefining Grünenthal’s organisation of product supply. As Head Global Operations (GO), Victor is accountable for Grünenthal’s product quality, cost and service to patients and customers worldwide. He leads around 2,000 people in the GO unit, spanning the full value chain of product supply, and is also accountable for Grünenthal’s Contract Manufacturing Business.
Leen Hofkens
Head Global Human Resources
Leen joined Grünenthal in 2018 and has driven a high-performance culture where individuals can thrive and make an impact on Grünenthal’s success. She was instrumental in launching the organisation’s Values & Behaviours, which guide our decision-making and help shape the culture at Grünenthal. Leen also played a key role in strengthening the Performance, Development and Compensation approach. She is also passionately driving Grünenthal’s Diversity and Engagement agenda and related activities.
Sebastian Köhler
General Counsel
Sebastian joined Grünenthal in 2018, bringing with him more than 10 years of expertise in executive roles and strategic legal consultancy, to build and lead the General Counsel area, which comprises Legal, Responsibility, Compliance, Enterprise Risk, Internal Audit, Patents and Trademarks, and Legal Operations. In his role, Sebastian ensures that Grünenthal receives best-in-class, in-house advice to support the sustainable implementation and evolution of its strategy. Examples include our strategic mergers and acquisitions such as the joint venture with Kyowa Kirin International in 2023.
Quentin Le Masne de Chermont
Head Corporate Strategy and Portfolio Management
Before joining Grünenthal in 2019, Quentin spent 8 years consulting companies in the healthcare sector on game-changing business strategies. His career began in research. He now drives our business goals at the intersection of Strategy, Commercial, R&D and Operations. Quentin has additional responsibility for deal assessment of established brand acquisitions.
Our transformational journey is progressing well, guided by a clear corporate strategy. Grünenthal’s business is in a uniquely strong position to continue its growth in the coming years.
Transformation Milestones
Financial growth
More than tripled company value, entered debt capital market and received favourable credit ratings.
R&D transformation
Built promising R&D pipeline with projects in all three Phases of clinical development and innovative pre-clinical platforms.
M&A
Since 2017, Grünenthal closed successful acquisitions of established brands and a Joint Venture with total deal value of more than € 2.1 billion, outperforming benchmark M&A in the pharmaceutical market.
Patient supply
Continued reliable supply of medicines despite strong headwinds in recent years.
Latin America
Focused promotion on innovative products in pain for better profitability and sustainable growth.
US presence
Fully represented in the USA with our research site Boston Innovation Hub and our commercial affiliate Averitas Pharma. In 2024, Grünenthal acquired US-based pharmaceutical company Valinor Pharma and its product MovantikTM. This expands Grünenthal’s portfolio of innovative treatments for patients with pain and related conditions and grows its presence in the US.
Inclusive culture & responsible business
Became a workplace with winning culture, ensured highest standards for conducting business responsibly.
Our strategy towards a World Free of Pain.
We are striving to achieve our vision by pursuing two key strategic approaches.
First, we are targeting organic growth by focusing our R&D activities on pain management.
Our strategy towards a World Free of Pain.
Second, we are tapping into inorganic growth by acquiring assets that strengthen our established brand portfolio – no matter which therapeutic area. These deals significantly boost our profitability, which enables us to continue investing in pain innovation.
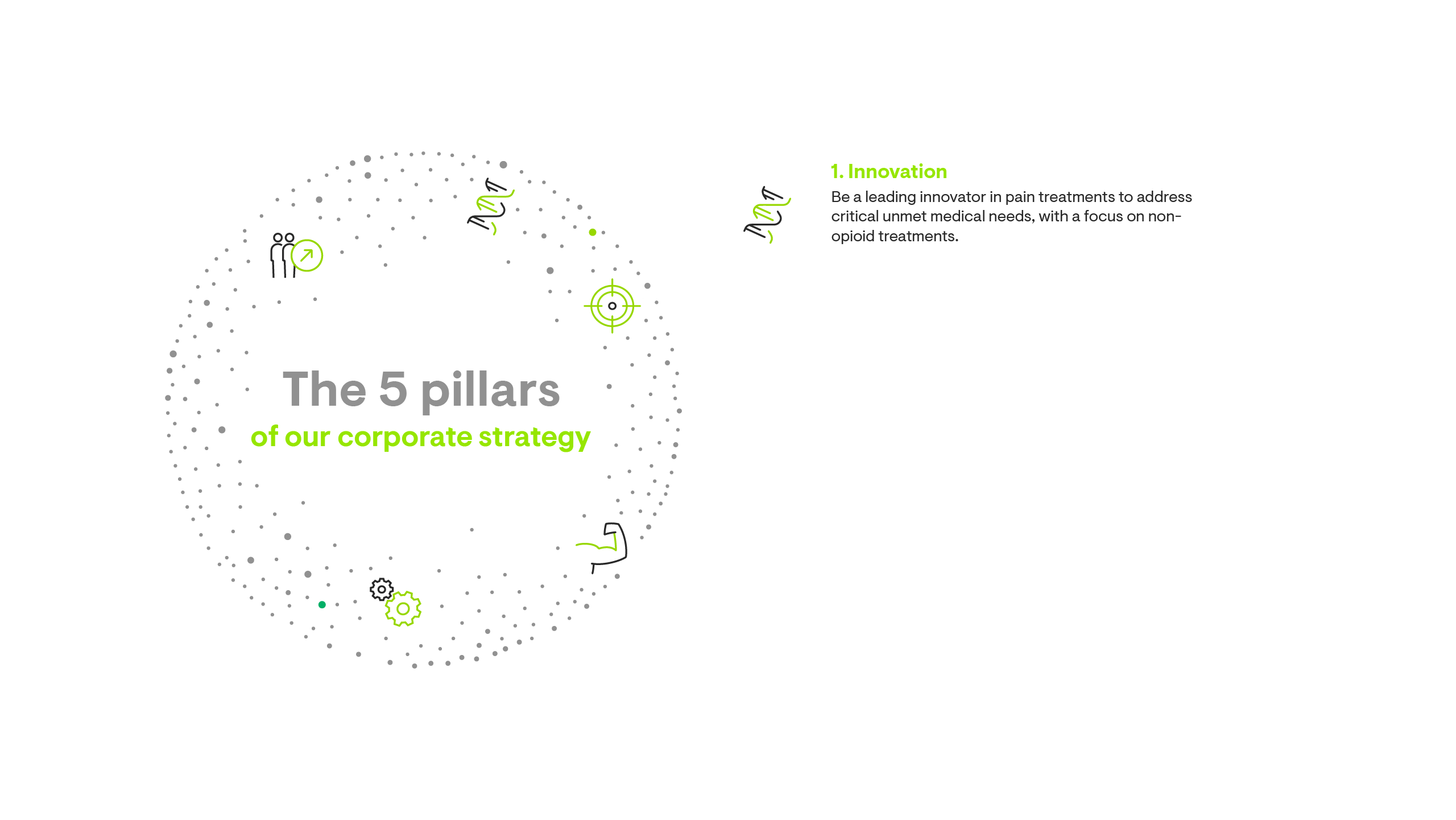
Our corporate strategy is built on five pillars.
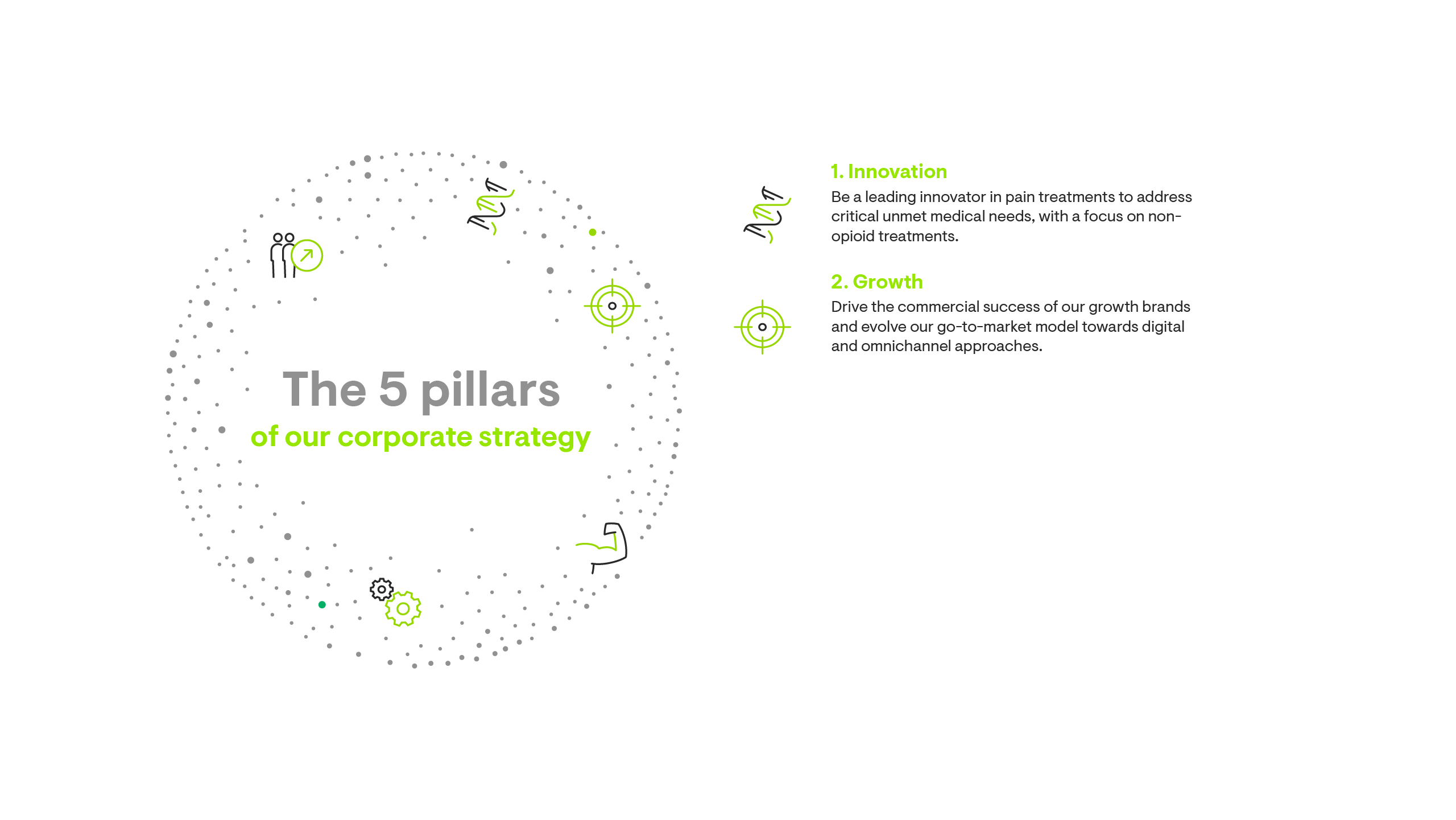
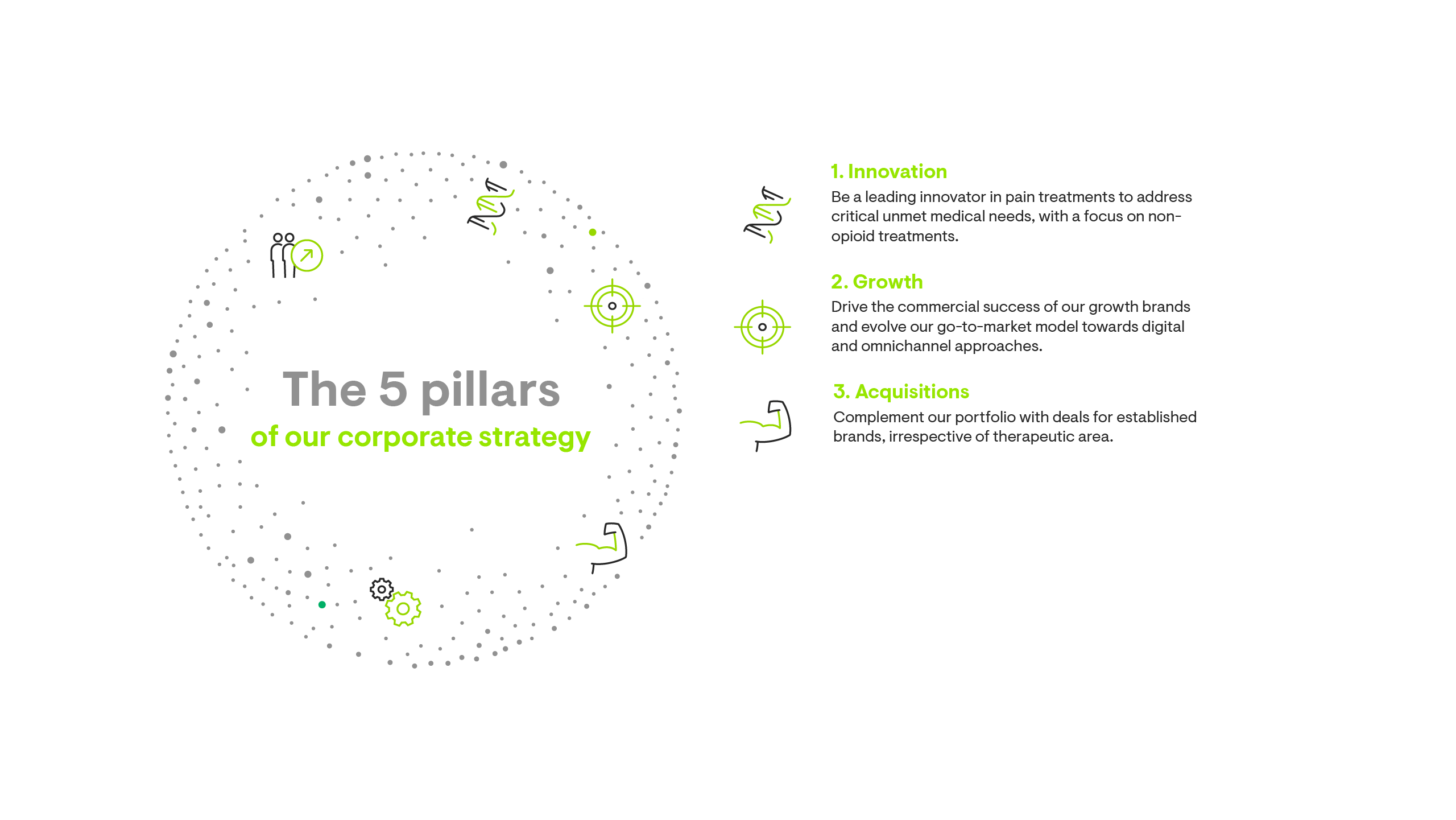
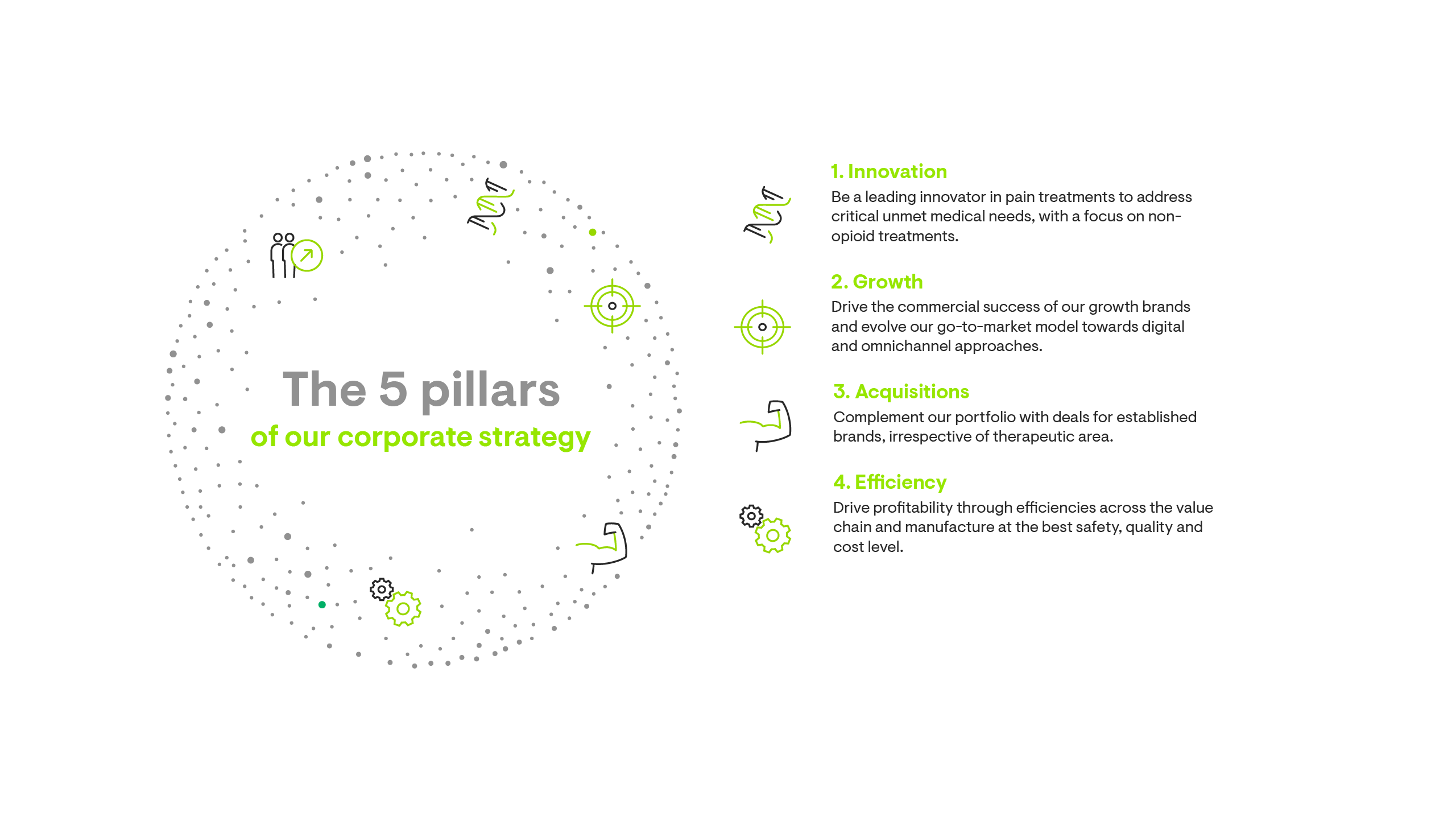
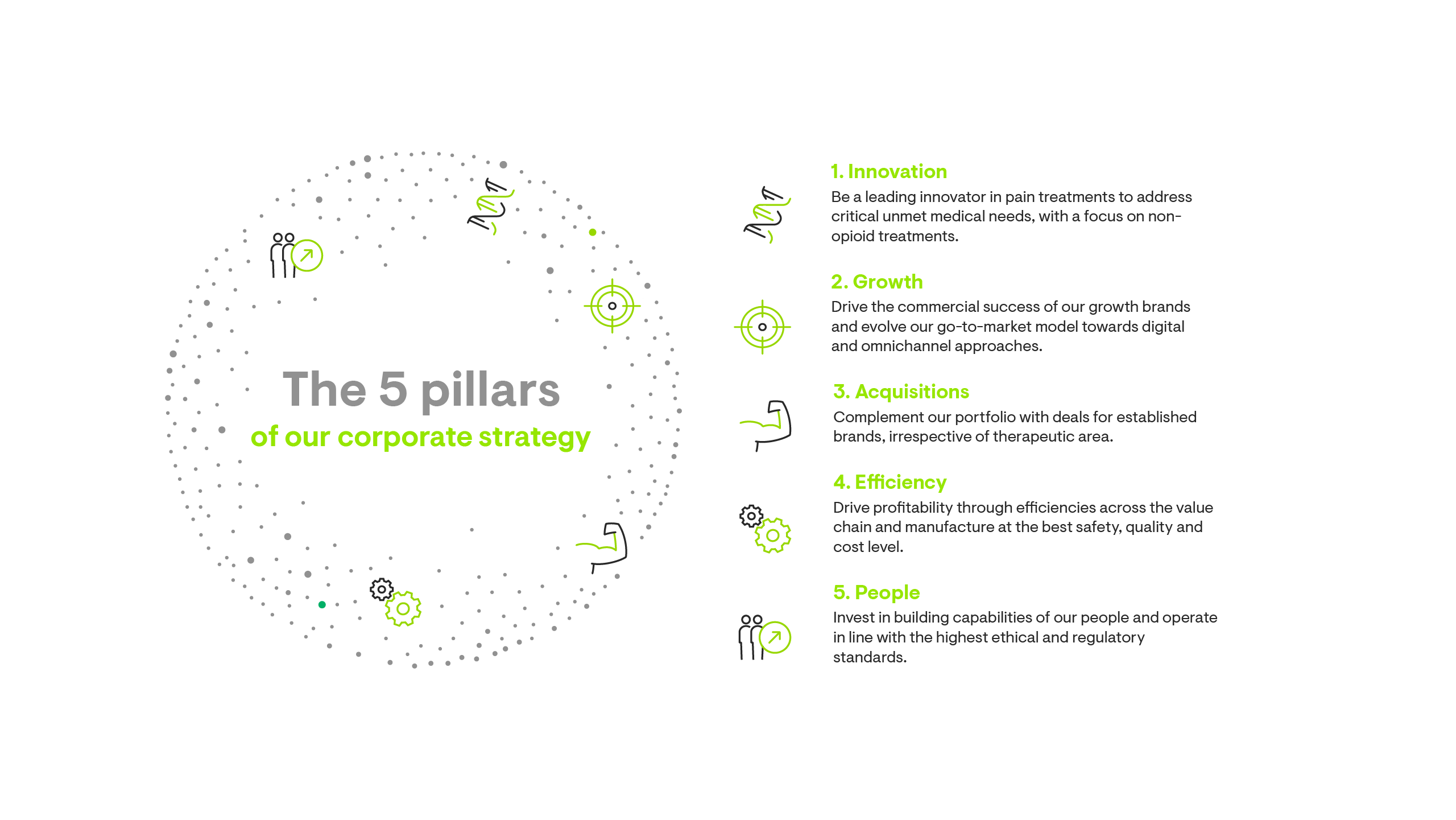
Growth through our M&A journey
Since 2017, Grünenthal has invested more than
€ 2.1 billion in successful deals that expand our
portfolio of products and R&D assets – while also
boosting our company’s profitability.
Financials
Our strategy continues to put us in a strong position to deliver outstanding business performance.
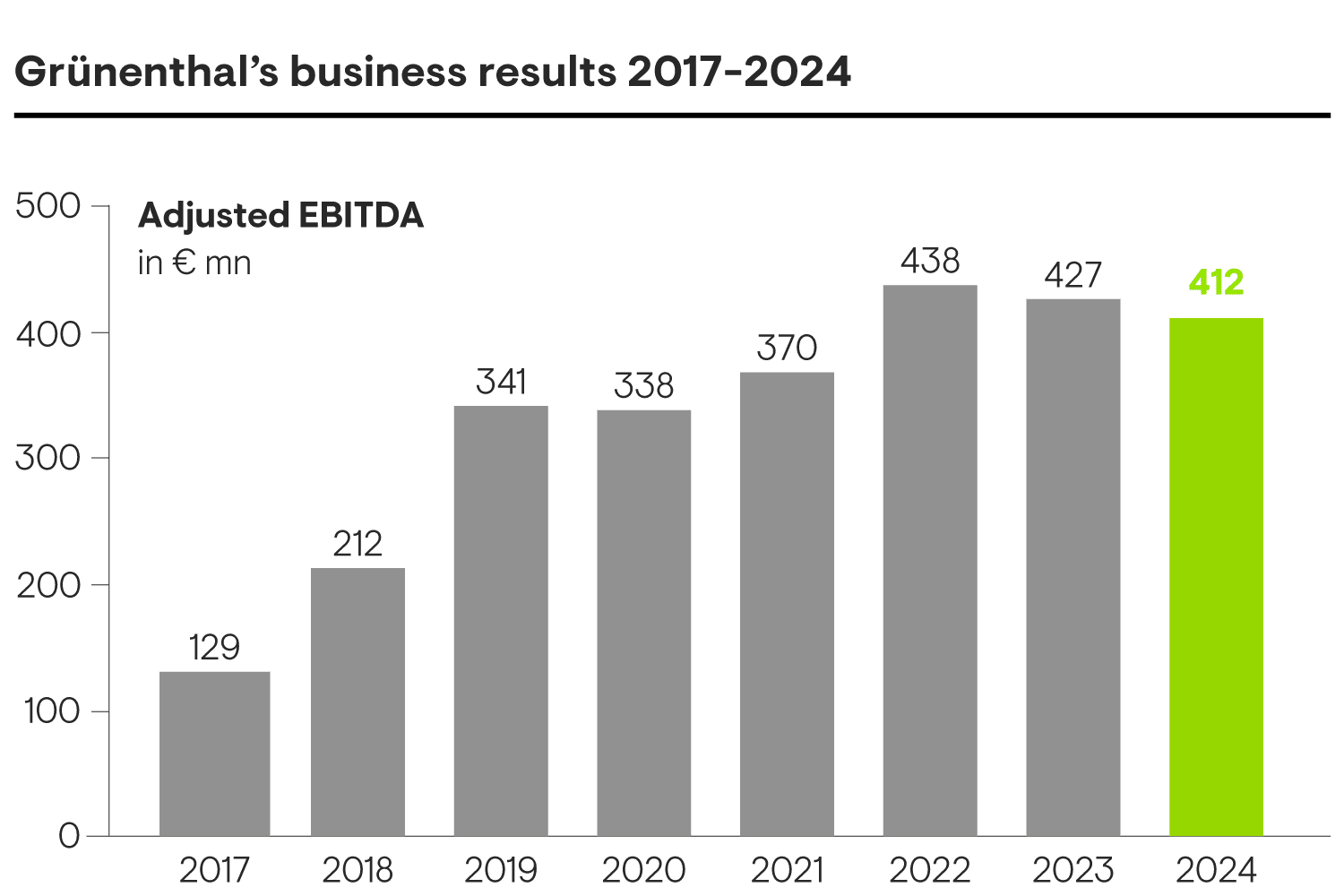
We maintained robust revenue of €1.8 billion in 2024, matching the prior year’s record level.
Adjusted EBITDA reached €412 million, and when considering the impact of PalexiaTM generics, this reflects an increase of +9% – all while strengthening our portfolio, increasing efficiency and advancing key R&D programmes.
Since 2017, we have more than tripled our EBITDA.
Strong Product Portfolio
Grünenthal’s product portfolio has a well-balanced and resilient mix of innovative growth brands and established medicines.
The growth brands are innovative and patent-protected products like QutenzaTM, as well as brands that continue to have valuable growth potential like VimovoTM and Movantik™/ MoventigTM.
The established medicines include all mature and off-patent products. They are characterised by high brand awareness, predictable and stable sales, and high profitability. Examples include NexiumTM, CrestorTM, NebidoTM and TramalTM.
Read our statement on the responsible use of opioid-based medicines here.
Cutting-edge Science
Existing pain therapies work for some patients – but not for all of them. That is why we engage in pioneering research to find next-generation, non-opioid pain medicines for patients in need.
Our R&D activities focus on four indications.
Peripheral neuropathic pain
Chronic low back pain
Osteoarthritis
Chronic post-surgical pain

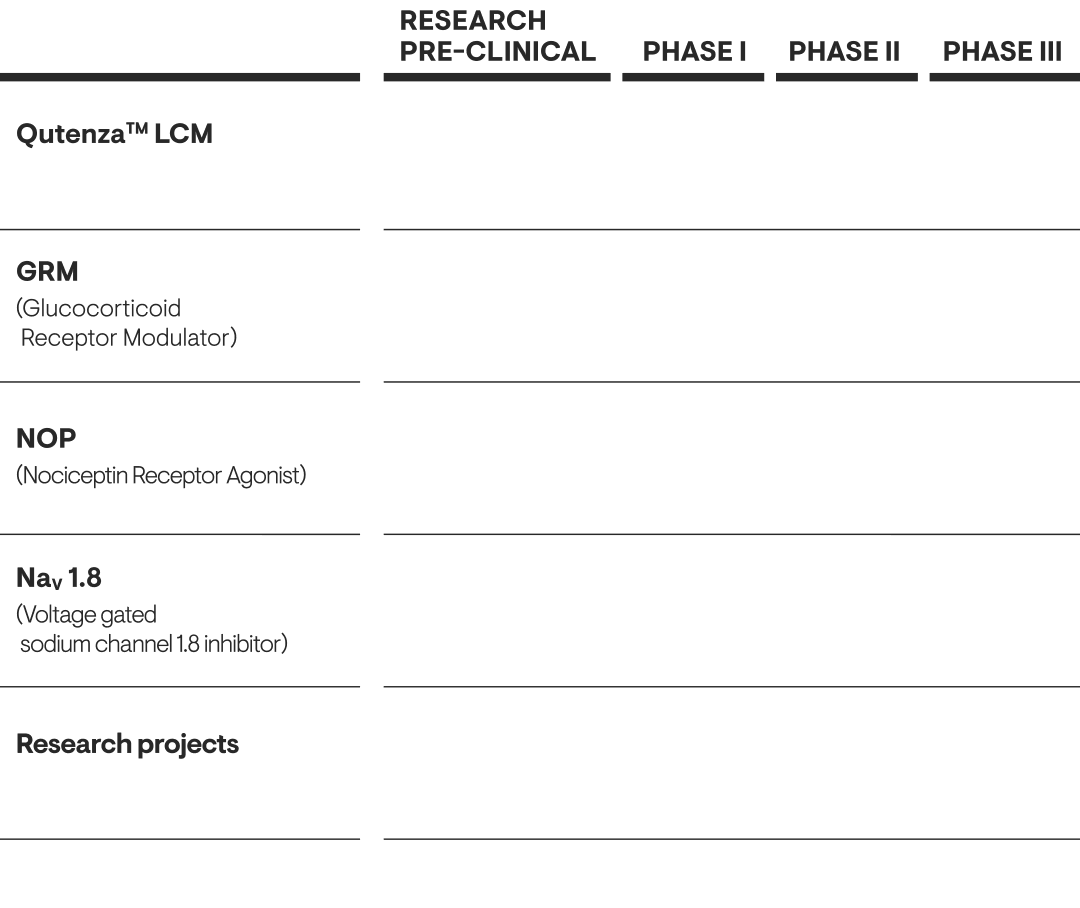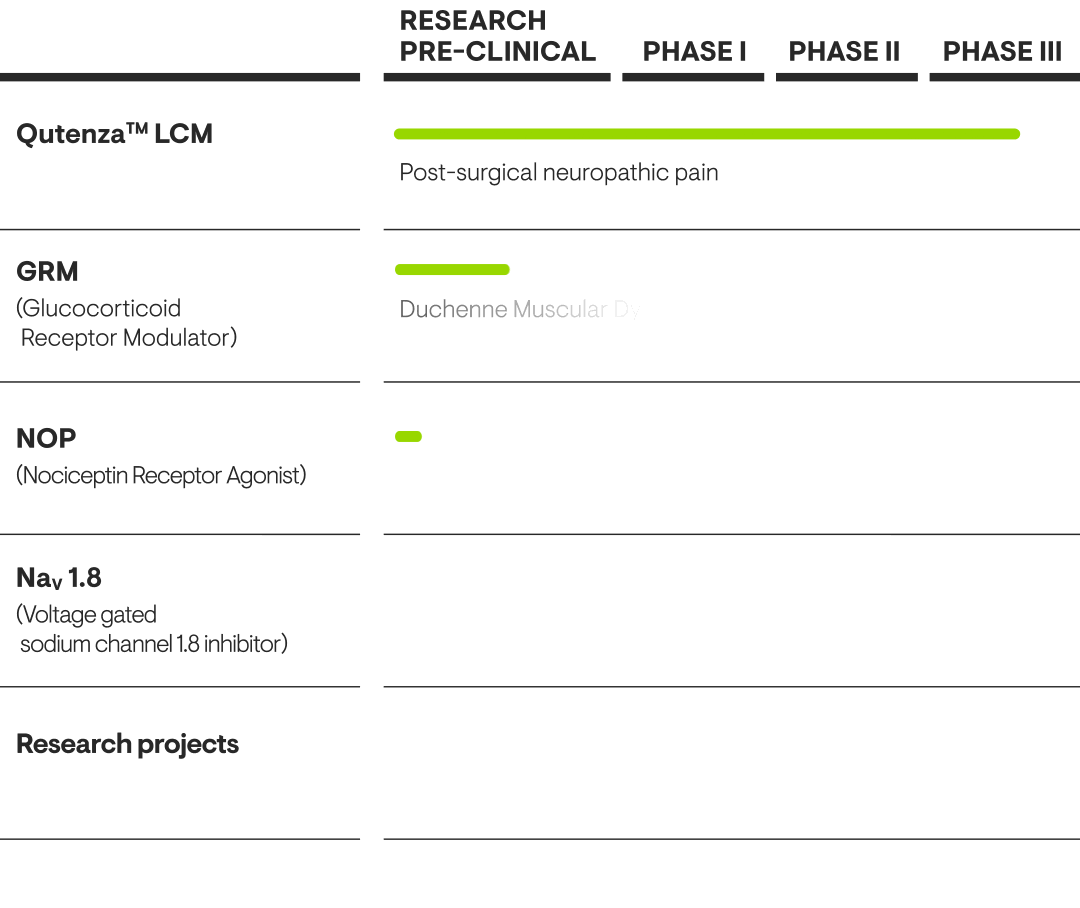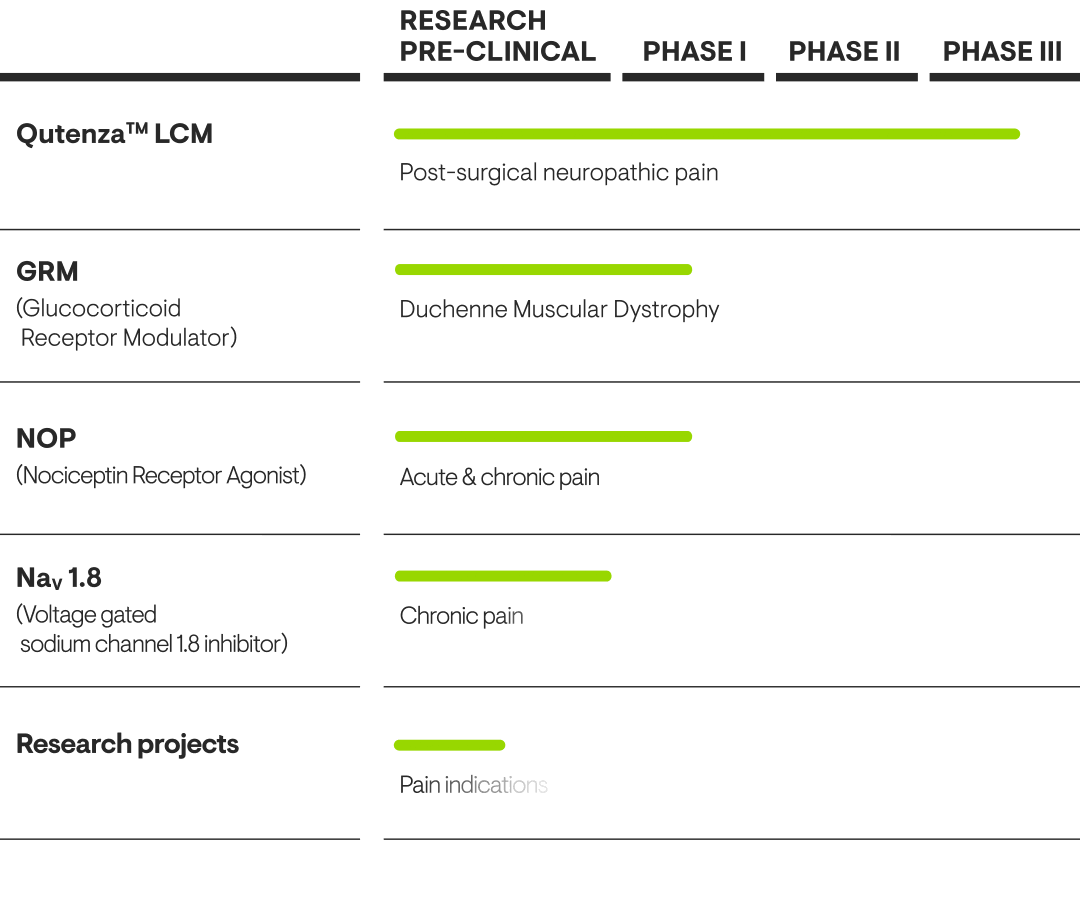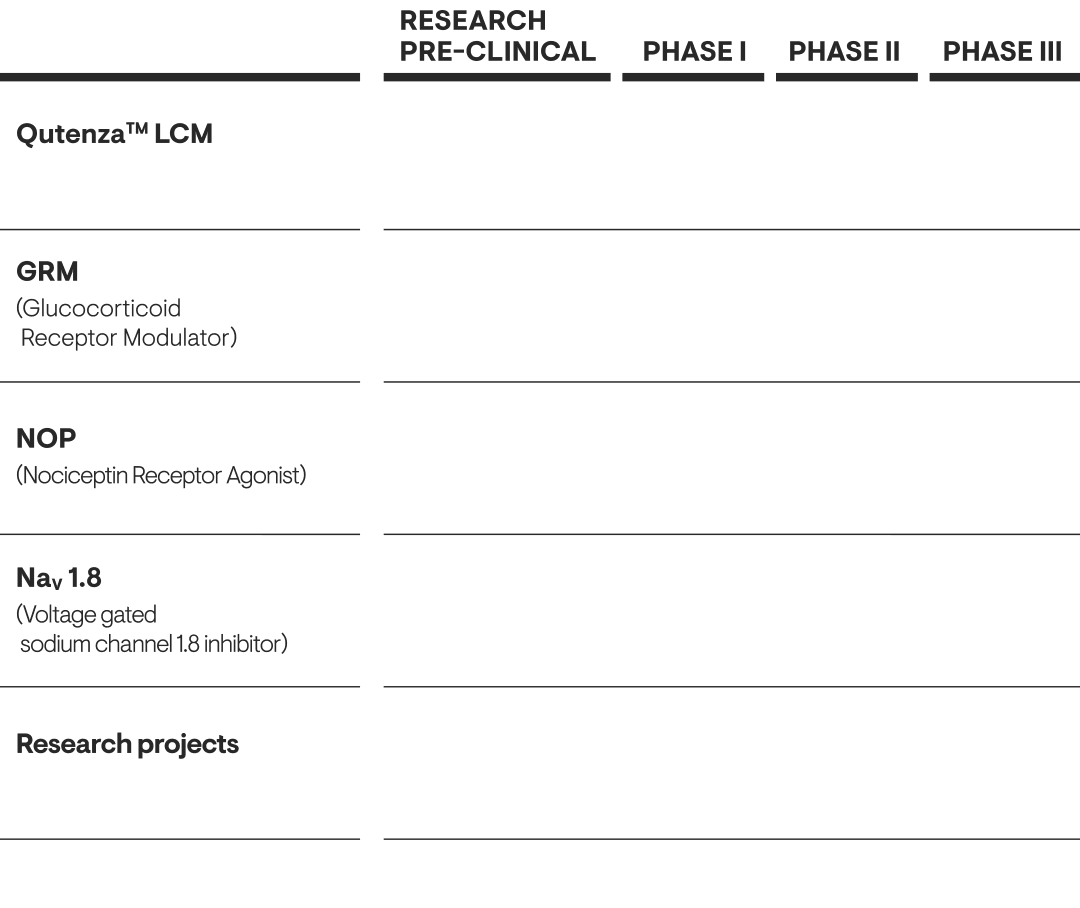
Our Pipeline
Our pharmaceutical scientists have created a leading pipeline of pain-related innovations.
LIFE CYCLE MANAGEMENT
Further developing Qutenza
Qutenza™ is a non-opioid treatment that can provide pain relief for several months. It contains prescription-strength capsaicin and is applied to the skin.
In Europe, Qutenza™ is approved for treating peripheral neuropathic pain. In the US, it is approved for treating peripheral neuropathic pain associated with post-herpetic neuralgia and for treating pain associated with diabetic peripheral neuropathy (DPN) of the feet in adults.
Grünenthal aims to make Qutenza™ more widely available by expanding the label – particularly in the US. A Phase III trial is investigating the efficacy, safety and tolerability of Qutenza™ in post-surgical neuropathic pain (PSNP), for example. We are also collaborating with partners to explore further potential indications.
OUR GRM PROGRAMME
Potential anti-inflammatory with improved safety
Tegacorat, our propriertary Glucocorticoid Receptor Modulator (GRM) is an oral investigational medicine developed to provide broad anti-inflammatory efficacy. It is also aiming to achieve a safety profile that allows longer-term treatments, which will address unmet medical needs and make an important difference to patients’ lives. Glucocorticoids, such as prednisolone, are known to be highly effective anti-inflammatory drugs. However, they come with several significant side effects, including reduced bone formation that may lead to osteoporosis, as well as increased glucose levels, which raises the risk of diabetes. These side effects are a strong limitation for the long-term use of glucocorticoids, despite their efficacy.
With our GRM programme, we are pursuing the development of clinical candidates for oral treatment with broad anti- inflammatory efficacy and the potential of significantly reduced side effects when compared to available glucocorticoid-based therapies. Tegacorat has concluded clinical Phase I with positive results and we are currently preparing a clinical Phase II trial in Duchenne Muscular Dystrophy (DMD).
Meet Florian Jakob, Grünenthal's Head Drug Discovery Engine, one of the passionate researcher behind our GRM programme.

OUR NOP PROGRAMME
Pioneering research for chronic pain
Our proprietary nociceptin (NOP) receptor agonists provide a unique mechanism of action for treating pain. They are predicted to deliver robust pain relief in a broad range of painful conditions without the side effects commonly associated with opioids.
For this reason, they may provide a unique and transformative first-in-class therapy option for patients living with pain.
The results of an ongoing clinical Phase I trial are expected in Q4 2025.
NaV – Creating the next generation of non-opioid pain medicines
One of Grünenthal’s most promising early research areas is our voltage-gated sodium channels (NaV) programme, where we are striving to create the next generation of non-opioid pain medicines. NaV channels can carry sodium ions into cells, resulting in an excitatory signal. If a channel's activity is modified so it can no longer carry sodium ions, it will also no longer be able to evoke excitatory signals.
Of the family of nine NaV channels, we are particularly interested in those expressed in dorsal root ganglion neurons (such as NaV 1.7, NaV 1.8 and NaV 1.9). These specific channels play roles in triggering excitatory signals in nociceptive neurones which are felt as pain by the human brain. As well as recognizing their key role in pain signalling, genetic and some clinical validation make them promising human pain targets. Manipulating these NaV channels in a way that suppresses or prevents their excitatory signalling could provide a significant analgesic effect across a range of chronic and acute pain conditions.
Grünenthal has created excellent, selective therapeutic candidates and we are preparing a first-in-human study for our lead candidate.
OUR RTX PROGRAMME
Researching a promising treatment for osteoarthritis
Resiniferatoxin (RTX) is an investigational medicine for the treatment of pain associated with osteoarthirits of the knee. The compound addresses the Transient Receptor Potential Vanilloid 1 (TRPV1) which plays a key role in the transmission of pain signals.
The ongoing clinical Phase III programme consists of three trials – two pivotal trials (KF7039-01 and KF7039-02) and an open-label safety study (KF7039-03). As primary endpoints, the pivotal trials evaluated the change in pain score on the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index. At the same time, the trials measured several secondary endpoints, including change in pain and physical function scores on the WOMAC Osteoarthritis Index.
Both pivotal trials did not meet their primary endpoints. Grünenthal will conclude this development programme with the completion of the open-label safety study KF7039-03 in the second half of 2025. To date, RTX has shown a favourable safety profile and has been well tolerated.

Boston Innovation Hub –
A centre of excellence for pain research
Our Innovation Hub in Boston is a centre of excellence for pain research, and an open invitation to scientists who are dedicated to addressing pain to team up with us as we work towards our vision of a world free of pain.
Greater Boston is recognised as the biggest biotech hub in the world, a centre for creating the next generation of pain medicines.
- > 1,000 biotech companies, as well as venture capital firms, small and big pharma.
- Leading academic and philanthropic institutes like the Massachusetts Institute for Technology (MiT) or the Broad Institute.
Science forward
Always exploring the latest science
R&D teams at Grünenthal are at the forefront of scientific progress within the pharmaceutical industry.
Our experts investigate ways for new technologies to open up transformative treatment options for patients. From leveraging single-cell methodologies through to unleashing the power of AI to accelerate our research activities, we are always embracing the possibilities of future-facing science.

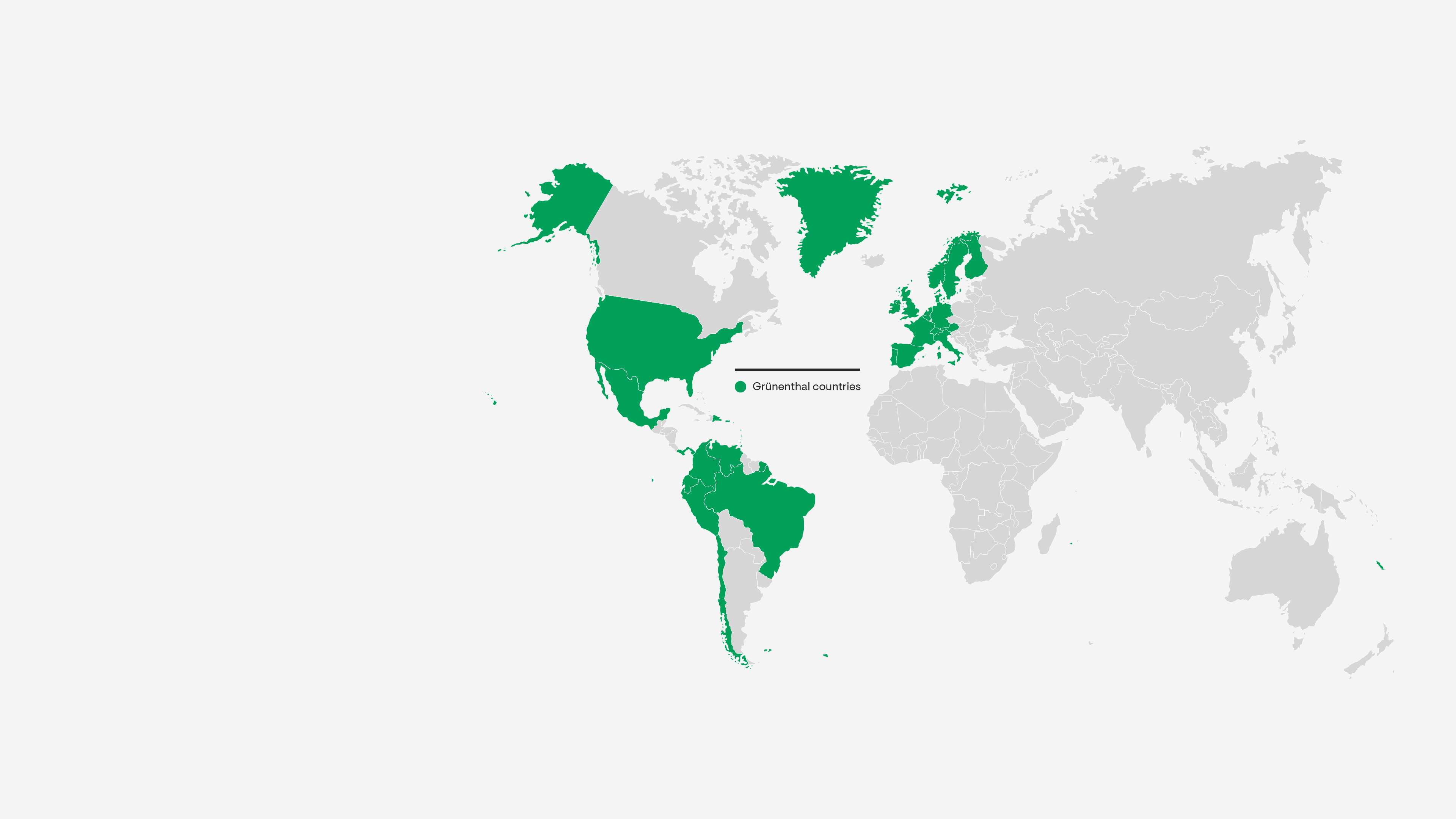
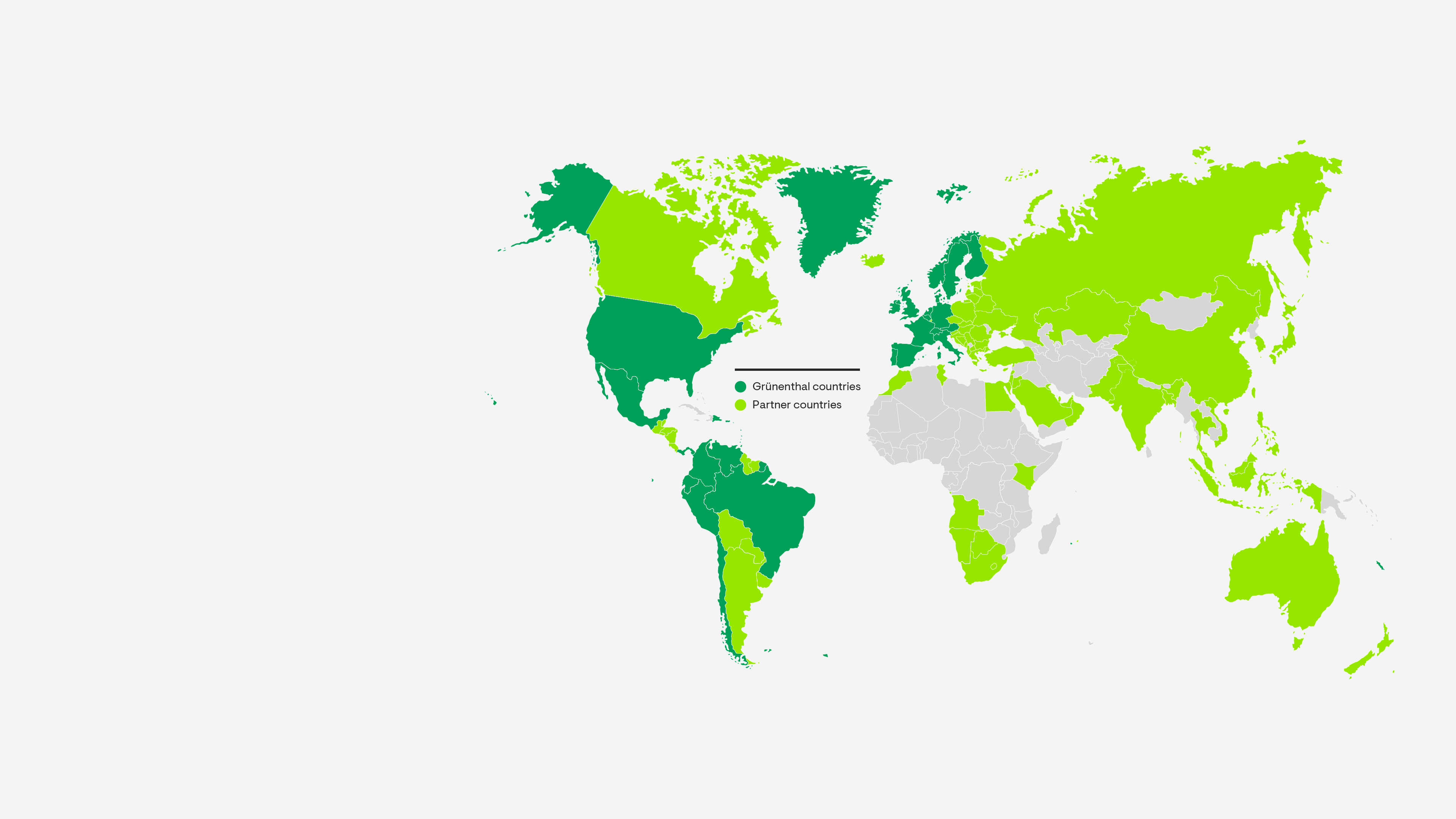
Market Presence
Reliable supply to patients
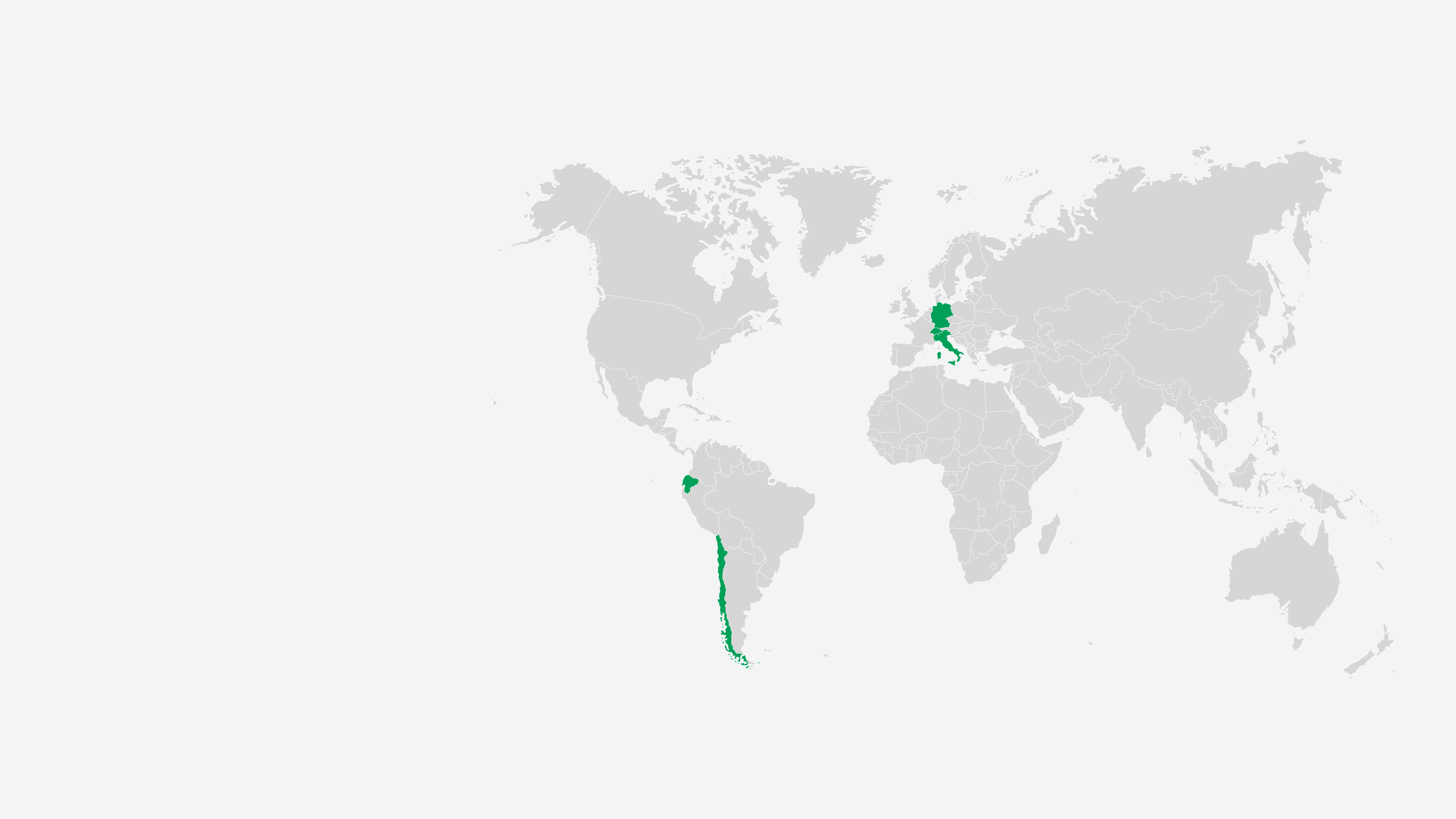
Our teams deliver a safe and reliable supply of pain treatments to patients in over 100 countries. We ensure the highest levels of safety, quality and cost-efficiency at every stage in our value chain.
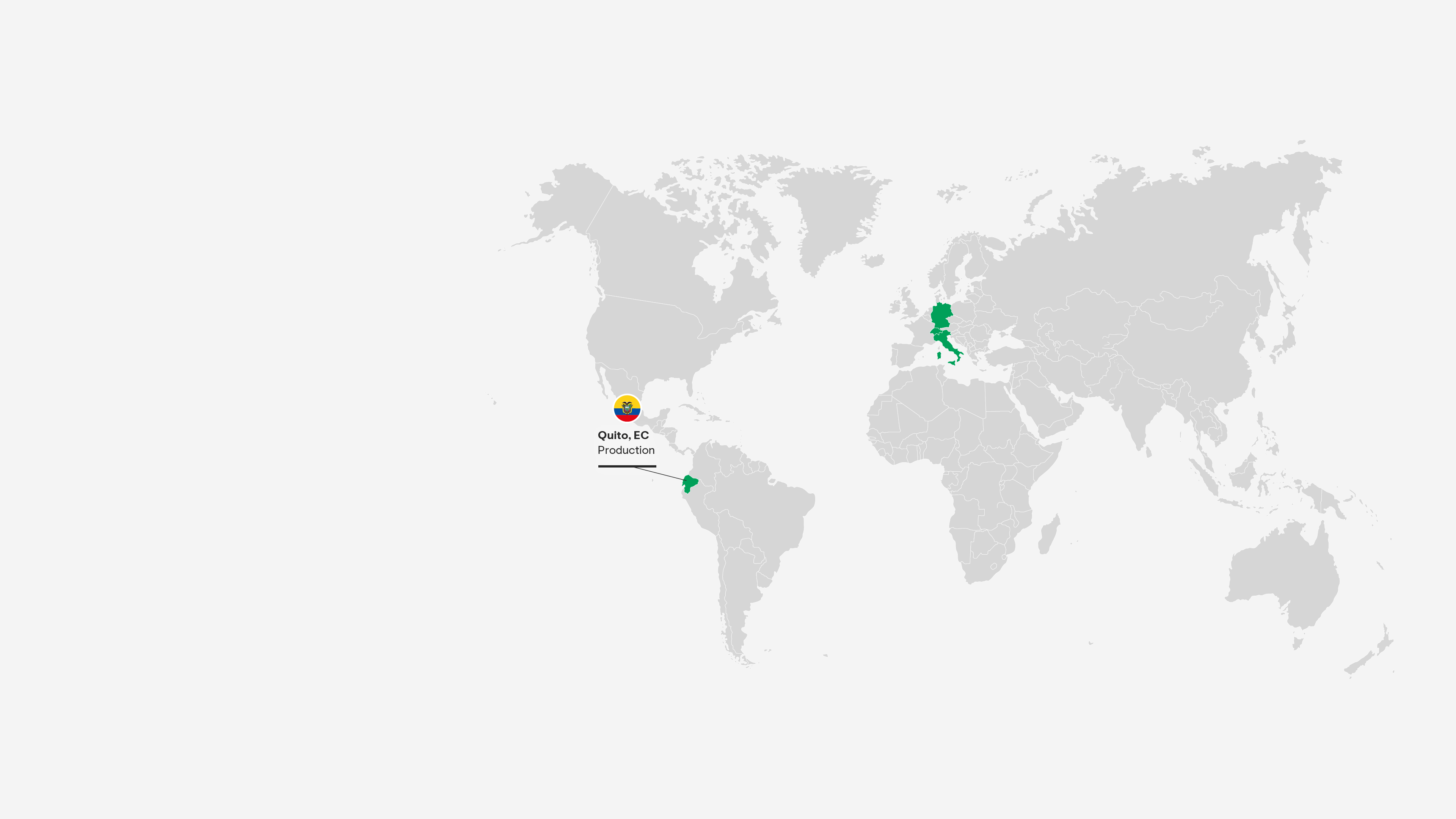
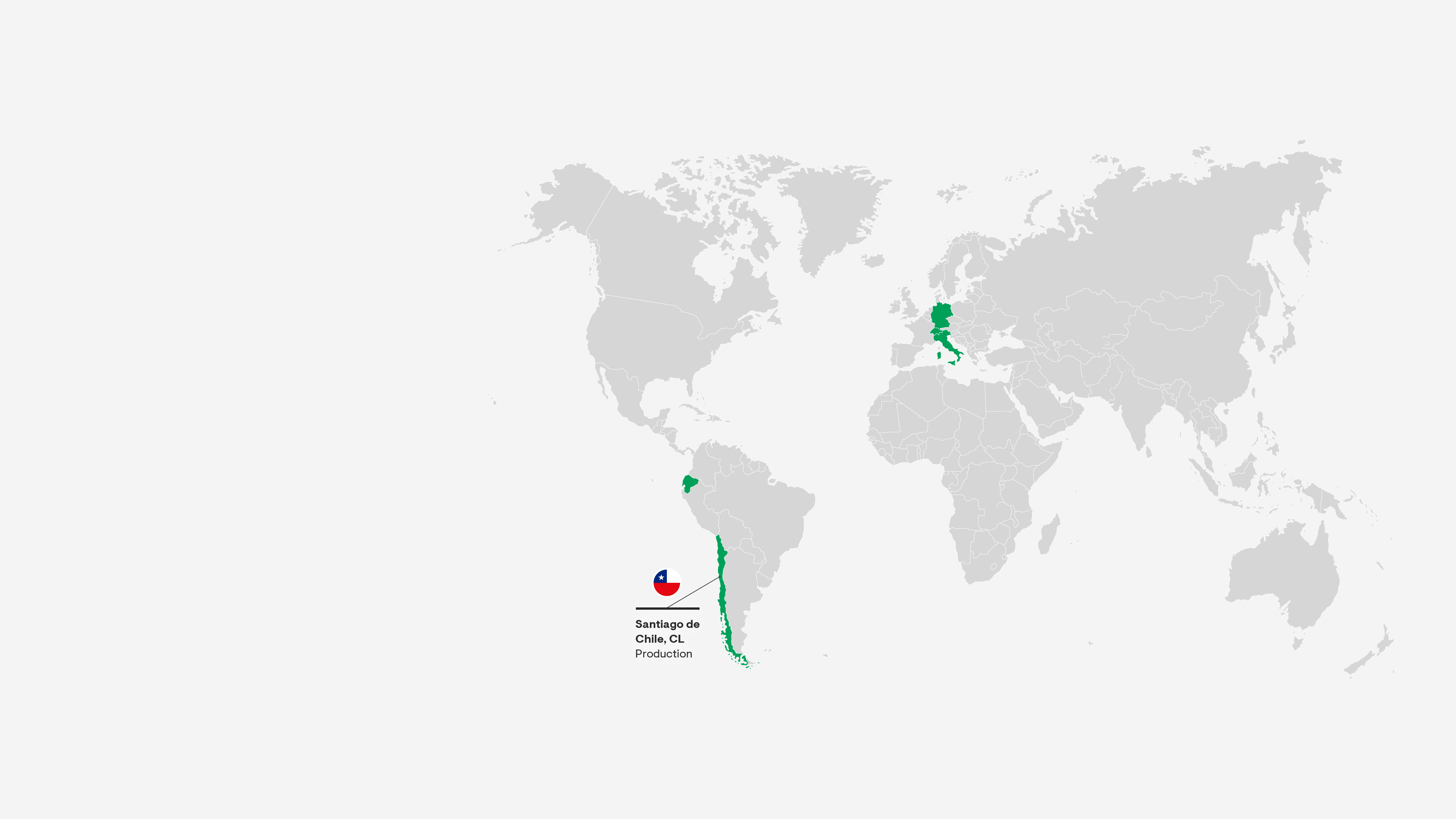
Grünenthal operates five production facilities – in Chile, Ecuador, Germany, Italy and Switzerland and provides a global production network with full service supply to 100 countries worldwide.
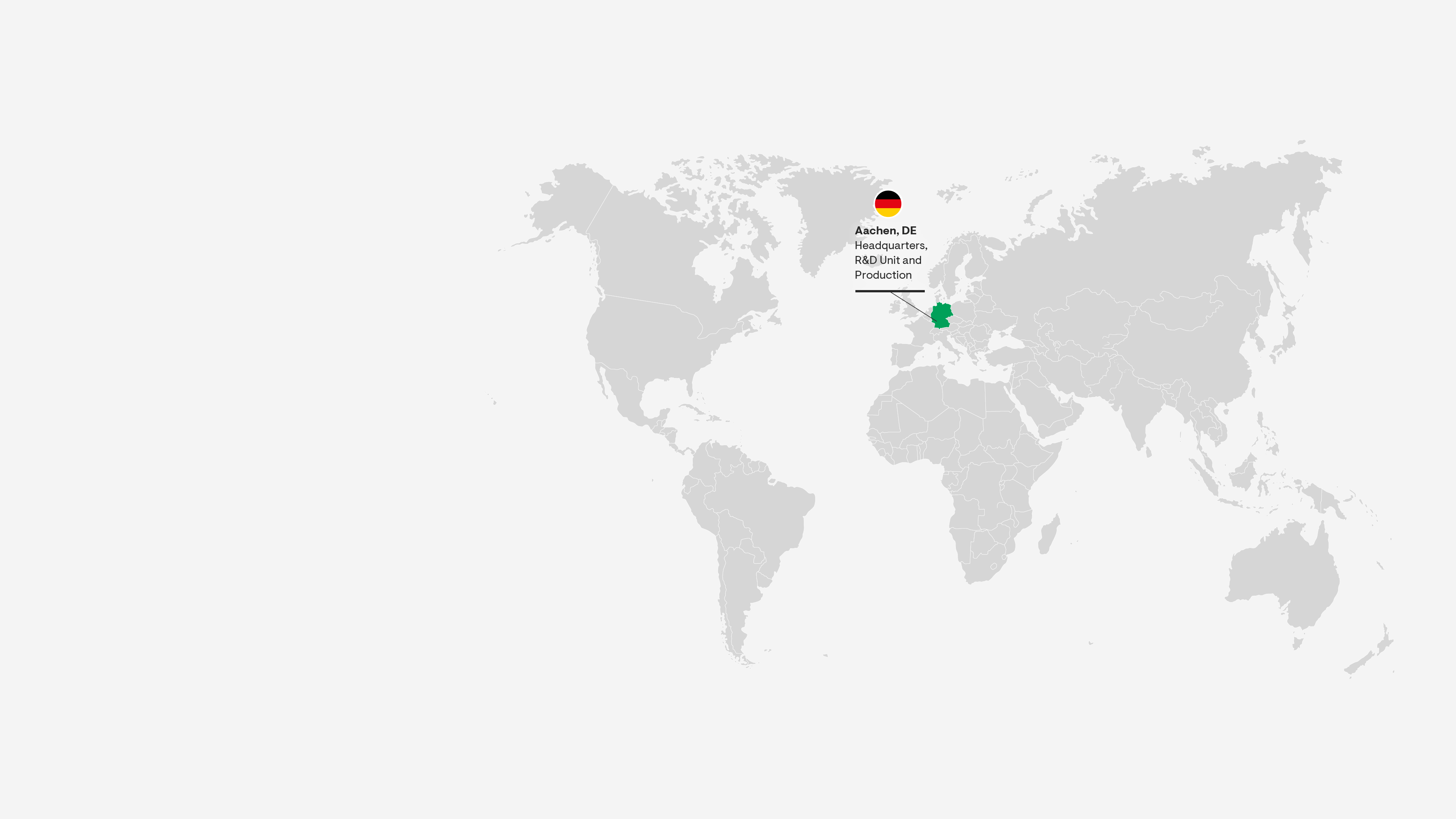
Aachen, Germany
Grünenthal is a global leader in pain management with its headquarters in Aachen, Germany. Our purpose is to change lives for the better – and innovation is our passion.
The scientists in Grünenthal’s state-of-the-art laboratories in Aachen are passionate about exploring innovative treatment options that have the potential to meaningfully improve the lives of patients around the world. Every day, Grünenthal’s scientists use their deep expertise to reimagine the future of pain management.
The Aachen site is our global center of excellence for pharma packaging and the main supplier of products to our affiliates, Contract Manufacturing Business, and partner businesses in over 80 countries.
Aachen also hosts one of Grünenthal's multi-purpose API plants, producing key portfolio products and medicines for external partners.
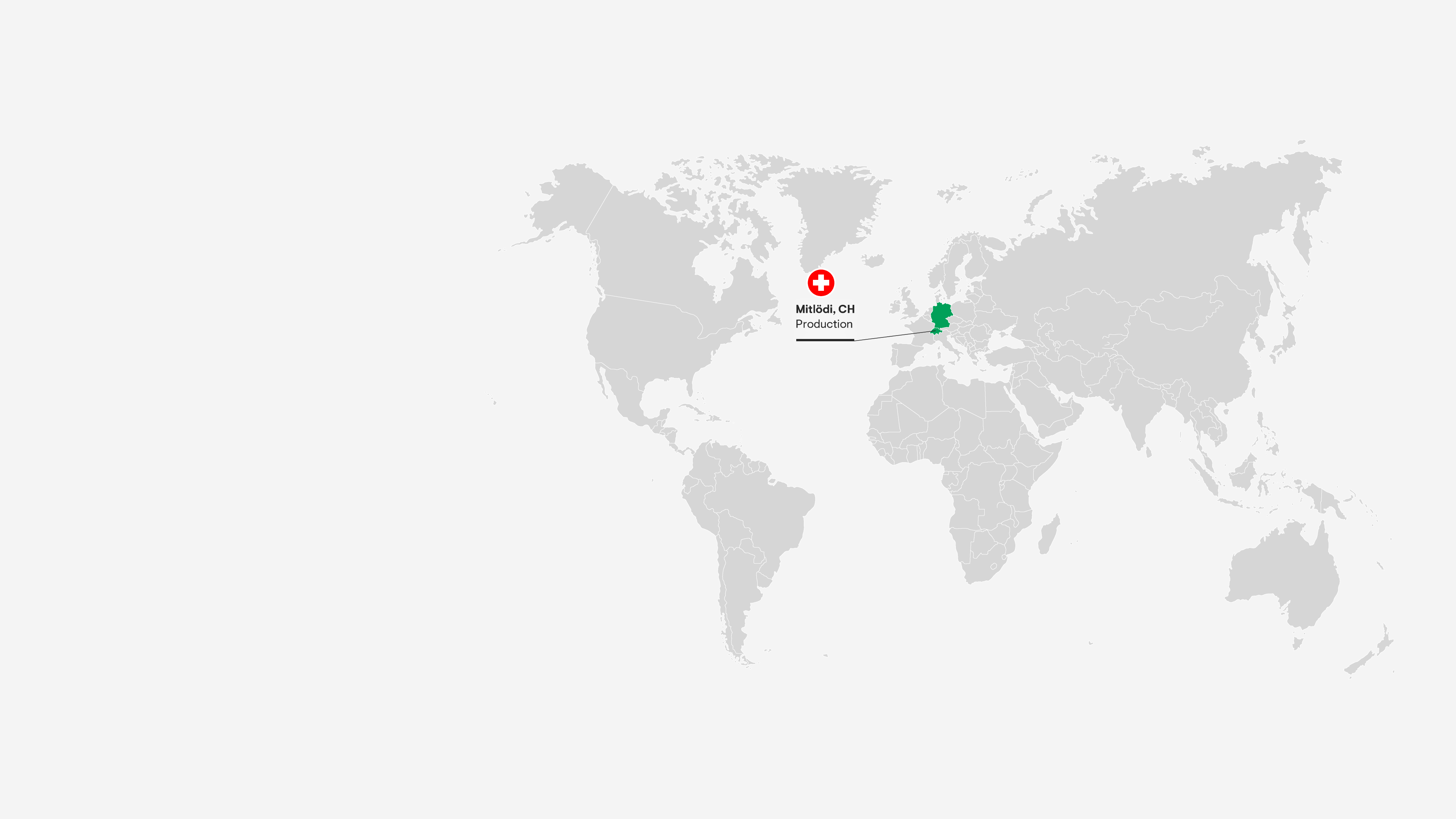
Mitlödi, Switzerland
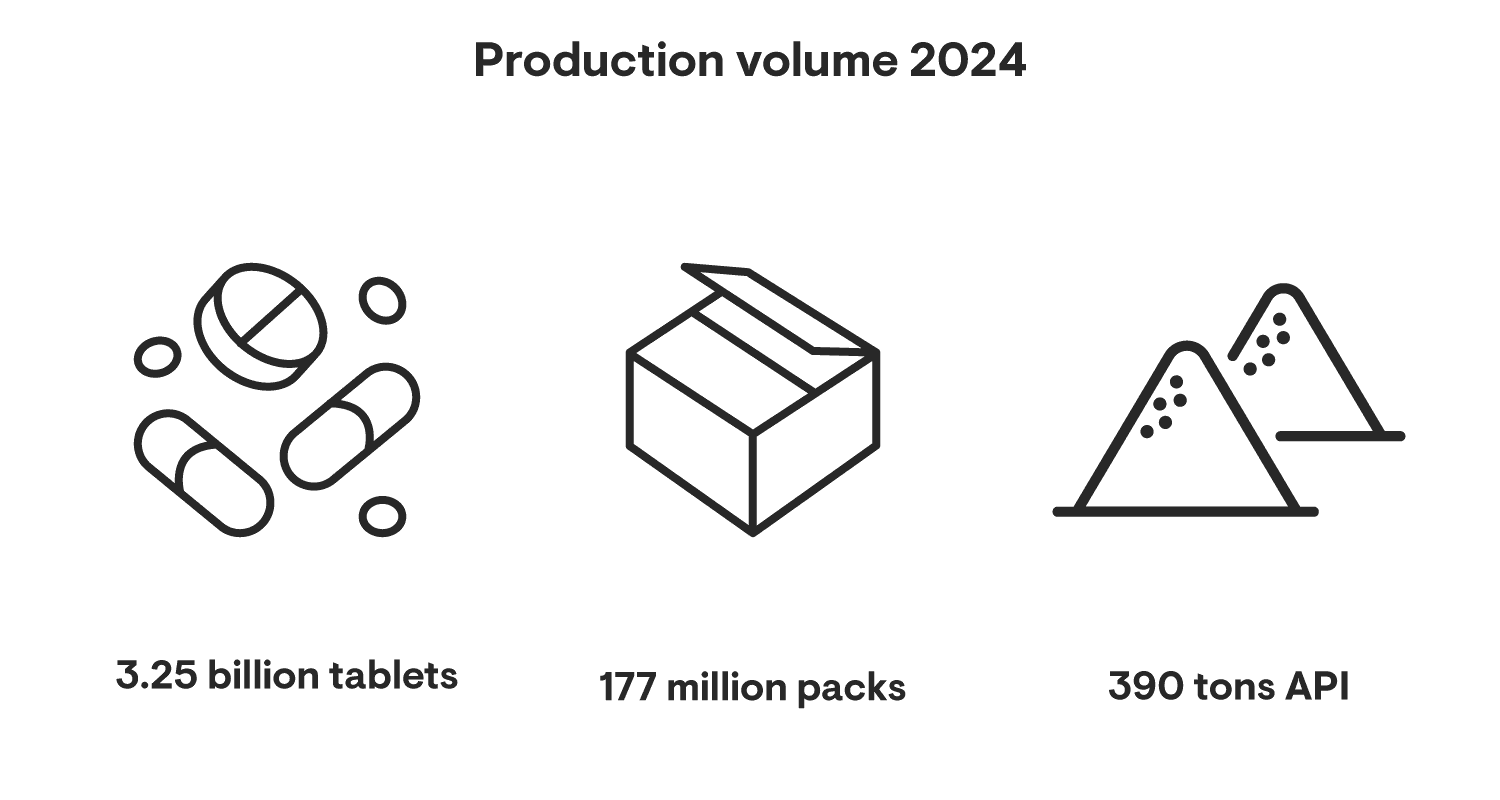
Our Active Pharmaceutical Ingredient (API) plant serves almost 20 countries and achieved record production volumes in 2024. It produces one-third of all tramadol worldwide – with 30 years of experience.
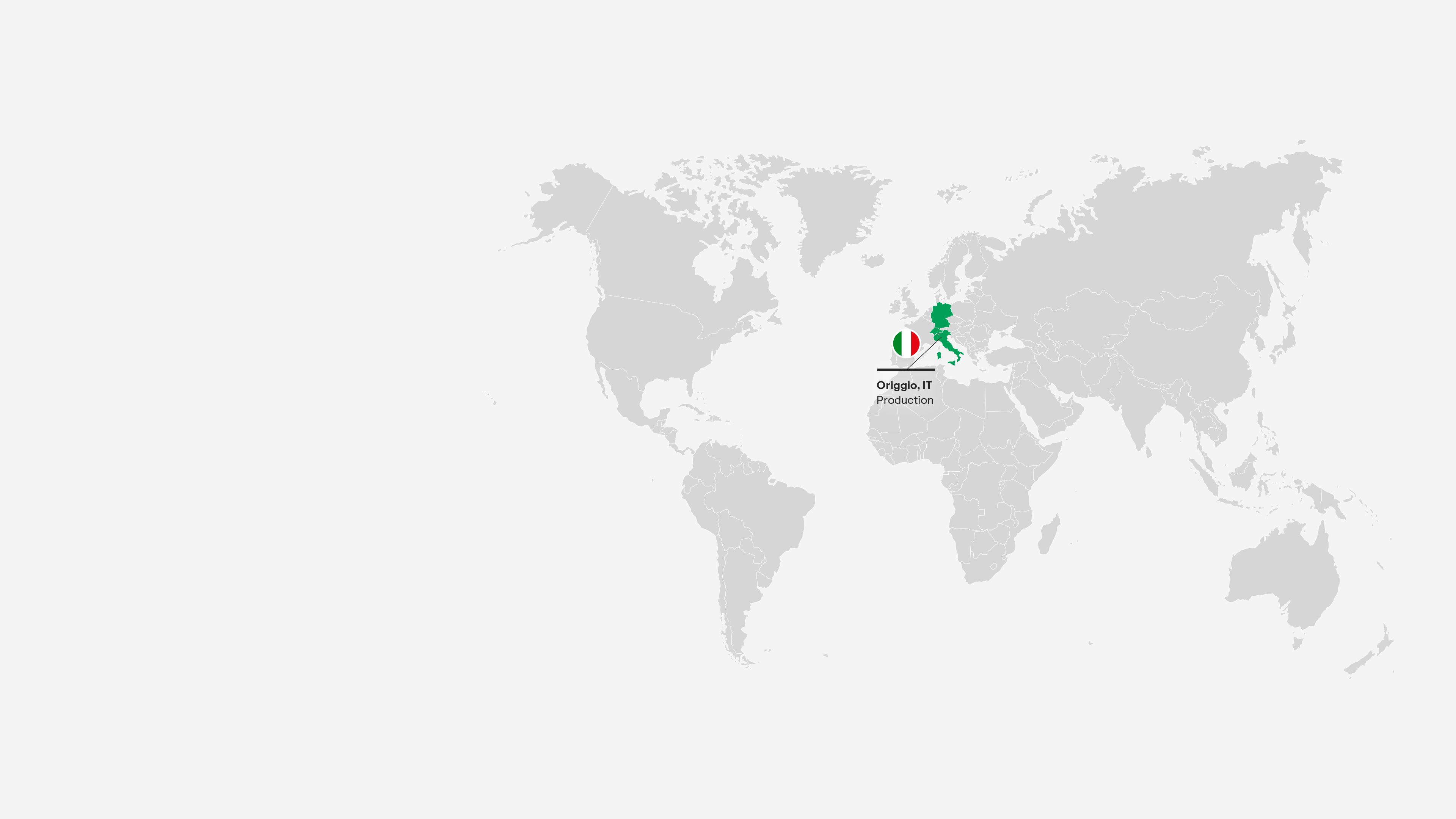
Origgio, Italy
This site plays a vital role in manufacturing our key brands for global markets, including the US and Japan. It offers a broad range of bulk production, with around 2.2 billion tablets and 23 million packs per year. It also supplies external customers, particularly with biopharma services and nasal spray technologies.
Quito, Ecuador
This site is one of Latin America’s largest and most advanced pharmaceutical production facilities, servicing countries across Latin America and Europe. Adhering to world-class quality standards, it produces solids, liquids, and semi-solids for both Grünenthal and our partners.
Santiago, Chile
This site is home to our centre of excellence for hormone production, which supplies products for patients across Latin America. The state-of-the-art technology at our second facility for conventional product manufacturing and packaging positions us among pioneers in Chile and the region.
Reliable Supply to Patients
We are proud that we successfully maintained an uninterrupted supply of medicines throughout 2024, despite a number of local and global challenges and while integrating various acquisitions into our company.
This applies not only to Grünenthal’s products but also to those of our partners. Customers value our proven reliability. We build trusted, long-term relationships that our partners can depend on, even in challenging times.
In 2024, third-party manufacturing accounted for almost 50 percent of our production volume.

A recognised industry leader in ESG
Our industry-leading way of doing business responsibly is reflected in our external Environment, Social and Governance (ESG) ratings. Independent rating agencies regularly assess our performance in ESG criteria, such as greenhouse gas emissions, employee health and safety and corporate governance.
Read more about our ESG ratings:
MSCI | Sustainalytics | Ecovadis
Our focus areas: Patient, People, Planet
Grünenthal aims to positively impact society and the world we live in – with and beyond its core business. We have a deep commitment to our patients and their families, our employees, our customers and investors, and the communities in which we operate. We focus on creating sustainable value in the three areas that matter most to our stakeholders: Patient, People and Planet.
We are committed to serving the needs of patients where it matters most: We focus our activities on topics such as patient safety, product quality, enhancing patients‘ quality of life, fostering innovation in pain management, promoting the responsible use of opioids, and improving access to healthcare.


Grünenthal’s social responsibility towards its workforce encompasses fair working conditions, workplace safety and health protection, training and development and the merit-based promotion of diversity, inclusion and equal opportunities. We are certified as a Great Place to Work® in 20 countries.

Our employees work with suppliers, partners and customers to reduce CO2 emissions, save energy and resources in our own operations, and decrease waste across our entire value chain. We are committed to the Science Based Targets initiative (SBTi), guiding our decarbonisation and
emissions reduction efforts.
Sustainability highlights from 2024:


Employees across the organisation dedicated more than 4,000 hours of their time to community volunteering in 2024 during our Grünenthal Gives initiative.
Grünenthal is a unique place to work – a mid-sized, science-driven company that is on a journey.
Every employee plays a big role in helping to achieve our shared goals. We believe it takes a team to truly change lives for the better.
Our employees have a real impact on patients and on the results we achieve – in our labs, in our manufacturing sites, in our offices and when interacting with healthcare professionals.
20 Grünenthal entities are certified as a Great Place to Work© – which covers almost all of our affiliates worldwide.
Meet our people and their passion for our vision.





"Our commitment to making pain more manageable is more than just a slogan; it's personal."
Magdalena Poznanski
Transaction Manager & Due Diligence Lead
"I see chronic pain as a barrier to unlocking human potential."
Omar Mossad
Disease Biology Scientist
"The work we do can significantly affect an individual, and that's what makes it exceptionally meaningful to me."
Laura Wasser
Sourcing Manager DACH
"I work towards a future where personalized pain medicine is a reality."
Andrew Lockhart
Head Translational and Disease Understanding Unit
"Given the complexity of our healthcare systems, making pain treatment more accessible to patients is a very fulfilling task."
Loi Vo
National Market Access Manager
As a privately-owned and science-driven pharmaceutical company, we have 4,300 employees working in labs, offices, factories and customer-facing field roles in 28 countries. Guided by our Values & Behaviours, every individual is encouraged to innovate in every possible way.
1 Oliveira DS, et al. Pain Medicine. 2019;20(4):736–746. | Nijs J, et al. PMR 2020;410-419 | Breivik H, et al. Eur J Pain. 2006;10(4):287–333
2 Treede RD, et al. Pain. 2015 Jun;156(6):1003-1007
3 Pain Alliance Europe, Survey on Chronic Pain 2017, Diagnosis, Treatment and Impact of Pain. Available at: https://www.pae-eu.eu/wp-content/uploads/2017/12/PAE-Survey-on-Chronic-Pain-June-2017.pdf. [Accessed April 2024]

Imprint.
Contact
Maren Thurow
Head of Global Communications
E-mail: GlobalCommunication@grunenthal.com
Photo Credits
Grünenthal, Image Professionals GmbH, Adobe Stock, Getty Images, Freepik.com
Disclaimer
Privacy
Publisher
GRÜNENTHAL PHARMA GMBH & CO. KG
Zieglerstrasse 6
52078 Aachen
Germany
Phone: +49 241 569 0
E-mail: info@grunenthal.com
www.grunenthal.com